Chemical Resistance List 2014
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
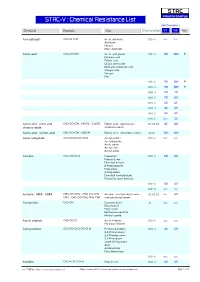
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Spill Containment - Chemical Resistance Charts
Spill Containment - Chemical Resistance Charts Max ˚C: Maximum temperature allowed. NR: Chemical is not recommended for use with our standard product. Please contact us for further information and quotation. Notes - Numbers: for some chemicals a different finish to the product may be required for extra protection. Please contact us for further information and quotation. Chemical Concentration Max ˚C Notes Chemical Concentration Max ˚C Notes Acetaldehyde 100 NR Alkylphenolpolyglycolether all 25 Acetic acid 10 95 0 Alkylphenolpolyglycolether sulphates Acetic acid 15 95 0 and Salts all 60 Acetic acid 25 95 0 Alkylsulfonate all 60 Acetic acid 40 80 Alkylsulfonic acid and sulfonates all 60 Acetic acid 50 70 allyl alcohol 100 NR Acetic acid 75 60 allyl chloride all NR 9 Acetic acid 80 45 Alpha methylstyrene 100 NR Acetic acid 85 45 Alum all 95 0 Acetic acid 100 NR Aluminium chloride all 95 0 Acetic acid glacial 100 NR Aluminium chlorohydrate all 95 0 Acetic anhydride 100 NR 9 Aluminium chlorohydroxide 50 95 0 Acetone 5 80 Aluminium citrate all 95 0 Acetone 10 80 Aluminium fluoride all 45 2 Acetone 100 NR Aluminium hydroxide all 70 2 Acetone: Aluminium nitrate all 95 0 Methylethyl ketone: 2:2:2 - Aluminium potassium sulphate all 95 0 Methylisobutyl ketone Aluminium sodium sulphate all 95 0 Acetonitrile all NR Aluminium sulphate all 95 0 Acetyl chloride 100 NR Aluminium sulphate/Acetic acid all 80 9 Acrylamide 50 - Amino acids all 40 Acrylic acid 25 45 Aminosulphonic acid all 80 Acrylic acid 100 NR Ammonia (dry gas) 100 40 Acrylic Latex all 80 -

Flowchem VE Corrosion Protection 2014
General Overview Flowchem VE Corrosion Resistance Guide Chemical Environment Recommended Temperature Limit °C WATER 100 ACIDS INORGANIC 100 ORGANIC 100 OXIDISING 65 ALKALINE INORGANIC 70 ORGANIC 50 SALTS INORGANIC 100 ORGANIC 50 SOLVENTS ALKYL, >C6 80 AROMATIC 25 ALCOHOLS 65 AMINES 25 HALOGENS 25 COPY KETONES NR NOTES: 1. General overview, exceptions within each group are possible. However increasing the molecular weight of chemicals will generally increase the service temperature. 2. This information is just a global grouping. Service temperature within one family can vary between different chemicals. 3. All temperatures are given for aqueous solutions. In case of concentrated mixtures service temperatures are generally lower. 4. Discoloration/ staining is not classified as chemical attack if hardness is unchanged. 5. Higher temperatures will reduce the chemical resistance shown in the performance table. 6. Some chemicals may concentrate due to evaporation and become more aggressive. 7. Mixtures of chemicals can be more aggressive than might be expected from the individual components alone. Note: The data contained herein is based on laboratory tests performed under carefully controlled conditions. No warranty can be expressed or implied regarding the accuracy of this information, as it will apply to actual operational use. Plant operations vary widely, and the individual results obtained are affected by the specific conditions encountered, which are beyond our control. Important: UNCONTROLLEDWe believe the information contained here to be true and accurate as of the date of publication. Flowcrete makes no warranty, expressed or implied, based on this literature and assumes no responsibility for consequential or incidental damages in the use of the systems described, including any warranty of merchantability or fitness. -

Hazardous Materials Management Plan
HAZARDOUS MATERIALS MANAGEMENT PLAN KENO HILL SILVER DISTRICT MINING OPERATIONS JULY 2018 HAZARDOUS MATERIALS MANAGEMENT PLAN KENO HILL SILVER DISTRICT MINING OPERATIONS Alexco Keno Hill Mining Corp. JULY 2018 TABLE OF CONTENTS 1 INTRODUCTION ................................................................................................................................................................ 1 2 STORAGE OF HAZARDOUS MATERIALS.................................................................................................................................. 2 2.1 UNDERGROUND CHEMICAL SUBSTANCES ........................................................................................................................... 3 3 HUMAN HEALTH AND SAFETY ............................................................................................................................................. 4 4 EMPLOYEE TRAINING ........................................................................................................................................................ 5 LIST OF TABLES Table 1-1 Hazardous Materials Management Plan Summary ....................................................................................... 1 Table 2-1 Summary of Reagents – Keno District Mill .................................................................................................... 3 Table 2-2 List of Fluids Used Underground ................................................................................................................... 4 LIST OF APPENDICES