Algorithms for Coherent Diffractive Imaging with X-Ray Lasers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction to Phasing Crystallography ISSN 0907-4449
research papers Acta Crystallographica Section D Biological Introduction to phasing Crystallography ISSN 0907-4449 Garry L. Taylor When collecting X-ray diffraction data from a crystal, we Received 30 August 2009 measure the intensities of the diffracted waves scattered from Accepted 22 February 2010 a series of planes that we can imagine slicing through the Centre for Biomolecular Sciences, University of St Andrews, St Andrews, Fife KY16 9ST, crystal in all directions. From these intensities we derive the Scotland amplitudes of the scattered waves, but in the experiment we lose the phase information; that is, how we offset these waves when we add them together to reconstruct an image of our Correspondence e-mail: [email protected] molecule. This is generally known as the ‘phase problem’. We can only derive the phases from some knowledge of the molecular structure. In small-molecule crystallography, some basic assumptions about atomicity give rise to relationships between the amplitudes from which phase information can be extracted. In protein crystallography, these ab initio methods can only be used in the rare cases in which there are data to at least 1.2 A˚ resolution. For the majority of cases in protein crystallography phases are derived either by using the atomic coordinates of a structurally similar protein (molecular replacement) or by finding the positions of heavy atoms that are intrinsic to the protein or that have been added (methods such as MIR, MIRAS, SIR, SIRAS, MAD, SAD or com- binations of these). The pioneering work of Perutz, Kendrew, Blow, Crick and others developed the methods of isomor- phous replacement: adding electron-dense atoms to the protein without disturbing the protein structure. -

System Design and Verification of the Precession Electron Diffraction Technique
NORTHWESTERN UNIVERSITY System Design and Verification of the Precession Electron Diffraction Technique A DISSERTATION SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT OF THE REQUIREMENTS for the degree DOCTOR OF PHILOSOPHY Field of Materials Science and Engineering By Christopher Su-Yan Own EVANSTON, ILLINOIS First published on the WWW 01, August 2005 Build 05.12.07. PDF available for download at: http://www.numis.northwestern.edu/Research/Current/precession.shtml c Copyright by Christopher Su-Yan Own 2005 All Rights Reserved ii ABSTRACT System Design and Verification of the Precession Electron Diffraction Technique Christopher Su-Yan Own Bulk structural crystallography is generally a two-part process wherein a rough starting structure model is first derived, then later refined to give an accurate model of the structure. The critical step is the deter- mination of the initial model. As materials problems decrease in length scale, the electron microscope has proven to be a versatile and effective tool for studying many problems. However, study of complex bulk structures by electron diffraction has been hindered by the problem of dynamical diffraction. This phenomenon makes bulk electron diffraction very sensitive to specimen thickness, and expensive equip- ment such as aberration-corrected scanning transmission microscopes or elaborate methodology such as high resolution imaging combined with diffraction and simulation are often required to generate good starting structures. The precession electron diffraction technique (PED), which has the ability to significantly reduce dynamical effects in diffraction patterns, has shown promise as being a “philosopher’s stone” for bulk electron diffraction. However, a comprehensive understanding of its abilities and limitations is necessary before it can be put into widespread use as a standalone technique. -

Optical Ptychographic Phase Tomography
University College London Final year project Optical Ptychographic Phase Tomography Supervisors: Author: Prof. Ian Robinson Qiaoen Luo Dr. Fucai Zhang March 20, 2013 Abstract The possibility of combining ptychographic iterative phase retrieval and computerised tomography using optical waves was investigated in this report. The theoretical background and historic developments of ptychographic phase retrieval was reviewed in the first part of the report. A simple review of the principles behind computerised tomography was given with 2D and 3D simulations in the following chapters. The sample used in the experiment is a glass tube with its outer wall glued with glass microspheres. The tube has a diameter of approx- imately 1 mm and the microspheres have a diameter of 30 µm. The experiment demonstrated the successful recovery of features of the sam- ple with limited resolution. The results could be improved in future attempts. In addition, phase unwrapping techniques were compared and evaluated in the report. This technique could retrieve the three dimensional refractive index distribution of an optical component (ideally a cylindrical object) such as an opitcal fibre. As it is relatively an inexpensive and readily available set-up compared to X-ray phase tomography, the technique can have a promising future for application at large scale. Contents List of Figures i 1 Introduction 1 2 Theory 3 2.1 Phase Retrieval . .3 2.1.1 Phase Problem . .3 2.1.2 The Importance of Phase . .5 2.1.3 Phase Retrieval Iterative Algorithms . .7 2.2 Ptychography . .9 2.2.1 Ptychography Principle . .9 2.2.2 Ptychographic Iterative Engine . -

Wide-Field, High-Resolution Fourier Ptychographic Microscopy
Wide-field, high-resolution Fourier ptychographic microscopy Guoan Zheng*, Roarke Horstmeyer, and Changhuei Yang Electrical Engineering, California Institute of Technology, Pasadena, CA 91125, USA *Correspondence should be addressed to: [email protected] Keywords: Ptychography; high-throughput imaging; digital wavefront correction; digital pathology Manuscript information: 11 text pages, 4 figures Supporting materials: 2 text pages, 8 figures, 1 video Abstract: In this article, we report an imaging method, termed Fourier ptychographic microscopy (FPM), which iteratively stitches together a number of variably illuminated, low-resolution intensity images in Fourier space to produce a wide-field, high-resolution complex sample image. By adopting a wavefront correction strategy, the FPM method can also correct for aberrations and digitally extend a microscope's depth-of-focus beyond the physical limitations of its optics. As a demonstration, we built a microscope prototype with a resolution of 0.78 μm, a field-of-view of approximately 120 mm2, and a resolution-invariant depth-of-focus of 0.3 mm (characterized at 632 nm). Gigapixel color images of histology slides verify FPM's successful operation. The reported imaging procedure transforms the general challenge of high-throughput, high- resolution microscopy from one that is coupled to the physical limitations of the system's optics to one that is solvable through computation. The throughput of an imaging platform is fundamentally limited by its optical system’s space- bandwidth product (SBP)1, defined as the number of degrees of freedom it can extract from an optical signal. The SBP of a conventional microscope platform is typically in megapixels, regardless of its employed magnification factor or numerical aperture (NA). -

Electron Ptychography Achieves Atomic-Resolution Limits Set by Lattice Vibrations
Electron ptychography achieves atomic-resolution limits set by lattice vibrations Zhen Chen1*, Yi Jiang2, Yu-Tsun Shao1, Megan E. Holtz3, Michal Odstrčil4†, Manuel Guizar- Sicairos4, Isabelle Hanke5, Steffen Ganschow5, Darrell G. Schlom3,5,6, David A. Muller1,6* 1School of Applied and Engineering Physics, Cornell University, Ithaca, NY 14853, USA 2Advanced Photon Source, Argonne National Laboratory, Lemont, IL 60439, USA 3Department of Materials Science and Engineering, Cornell University, Ithaca, NY, USA 4Paul Scherrer Institut, 5232 Villigen PSI, Switzerland 5Leibniz-Institut für Kristallzüchtung, Max-Born-Str. 2, 12489 Berlin, Germany 6Kavli Institute at Cornell for Nanoscale Science, Ithaca, NY, USA * Correspondence to: [email protected] (Z.C.); [email protected] (D.A.M.) †Present address: Carl Zeiss SMT, Carl-Zeiss-Straße 22, 73447 Oberkochen, Germany Abstract: Transmission electron microscopes use electrons with wavelengths of a few picometers, potentially capable of imaging individual atoms in solids at a resolution ultimately set by the intrinsic size of an atom. Unfortunately, due to imperfections in the imaging lenses and multiple scattering of electrons in the sample, the image resolution reached is 3 to 10 times worse. Here, by inversely solving the multiple scattering problem and overcoming the aberrations of the electron probe using electron ptychography to recover a linear phase response in thick samples, we demonstrate an instrumental blurring of under 20 picometers. The widths of atomic columns in the measured electrostatic potential are now no longer limited by the imaging system, but instead by the thermal fluctuations of the atoms. We also demonstrate that electron ptychography can potentially reach a sub-nanometer depth resolution and locate embedded atomic dopants in all three dimensions with only a single projection measurement. -

X-Ray Crystallography
X-ray Crystallography Prof. Leonardo Scapozza Pharmceutical Biochemistry School of Pharmaceutical Sciences University of Geneva, University of Lausanne E-mail: [email protected] Aim • Introduce the students to X-ray crystallography • Give the students the tools to “evaluate” a X-ray structure based scientific paper 1 Outline • The History of X-ray • The Principle of X-ray • The Steps towards the 3D structure – Crystallization – X-ray diffraction and data collection – From Pattern of Diffraction to Electron Density – X-ray structure quality assessment An extract of a structure paper 2.1. Crystallization • The hTK1 was cloned as N-terminal thrombin-cleavable His6–tagged fusion protein missing 14 amino acids of the N-terminus and 40 amino acids of the C–terminus of the wild type hTK1 sequence of 234 amino acids (this construct is further on called hTK1). The purified hTK1, consisting of residues 15-194 of the wild type sequence plus an N–terminal extension of 15 residues containing a His6–tag, was eluted from gel filtration column at a concentration of approximate 7 mg/ml with a buffer containing 5 mM Tris at pH 7.2, 10 mM NaCl and 10 mM DTT. For protein crystallization we used the hanging drop method at 23°C. Initial conditions for crystallization were found using Crystal screen Cryo no. 40 (Hampton Research). The protein solution was mixed in a 1:1 ratio with crystallization buffer (0.095 mM tri–sodium citrate pH 5.5, 12% PEG 4000, 10% isopropanol) to set up drops of 6 μl. The reservoir contained 500 μl of crystallization buffer. -

Near-Field Fourier Ptychography: Super- Resolution Phase Retrieval Via Speckle Illumination
Near-field Fourier ptychography: super- resolution phase retrieval via speckle illumination HE ZHANG,1,4,6 SHAOWEI JIANG,1,6 JUN LIAO,1 JUNJING DENG,3 JIAN LIU,4 YONGBING ZHANG,5 AND GUOAN ZHENG1,2,* 1Biomedical Engineering, University of Connecticut, Storrs, CT, 06269, USA 2Electrical and Computer Engineering, University of Connecticut, Storrs, CT, 06269, USA 3Advanced Photon Source, Argonne National Laboratory, Argonne, IL 60439, USA. 4Ultra-Precision Optoelectronic Instrument Engineering Center, Harbin Institute of Technology, Harbin 150001, China 5Shenzhen Key Lab of Broadband Network and Multimedia, Graduate School at Shenzhen, Tsinghua University, Shenzhen, 518055, China 6These authors contributed equally to this work *[email protected] Abstract: Achieving high spatial resolution is the goal of many imaging systems. Designing a high-resolution lens with diffraction-limited performance over a large field of view remains a difficult task in imaging system design. On the other hand, creating a complex speckle pattern with wavelength-limited spatial features is effortless and can be implemented via a simple random diffuser. With this observation and inspired by the concept of near-field ptychography, we report a new imaging modality, termed near-field Fourier ptychography, for tackling high- resolution imaging challenges in both microscopic and macroscopic imaging settings. The meaning of ‘near-field’ is referred to placing the object at a short defocus distance with a large Fresnel number. In our implementations, we project a speckle pattern with fine spatial features on the object instead of directly resolving the spatial features via a high-resolution lens. We then translate the object (or speckle) to different positions and acquire the corresponding images using a low-resolution lens. -

Coherent Diffraction Imaging
Coherent Diffraction Imaging Ian Robinson London Centre for Nanotechnology Felisa Berenguer Diamond Light Source Ross Harder Richard Bean Structural Biology Moyu Watari Oxford University March 2009 I. K. Robinson, STRUBI Mar 2009 1 Outline • Imaging with X-rays • Coherence based imaging • Nanocrystal structures • Extension to phase objects • Exploration of crystal strain • Biological imaging by CXD I. K. Robinson, STRUBI Mar 2009 2 Types of Full-Field Microscopy (Y. Chu) X-ray Topography Projection Imaging, Tomography, PCI Miao et al (1999) Coherent X-ray Diffraction Coherent Diffraction imaging Transmission Full-Field MicroscopyI. K. Robinson, STRUBI Mar 2009 3 Full-Field Diffraction Microscopy Lensless X-ray “Microscope” APS ξHOR= 20µm, focus to 1µm NSLS-II ξHOR= 500µm, focus to 0.05µm I. K. Robinson, STRUBI Mar 2009 4 Longitudinal Coherence Als-Nielsen and McMorrow (2001) I. K. Robinson, STRUBI Mar 2009 5 Lateral (Transverse) Coherence Als-Nielsen and McMorrow (2001) I. K. Robinson, STRUBI Mar 2009 6 Smallest Beam using Slits (9keV) 10 Beam 100mm away size (micron) 50mm away 20mm away 10mm away 0 0 10 Slit size (micron) I. K. Robinson, STRUBI Mar 2009 7 Fresnel Diffraction when d2~λD X-ray beam defined by RB slits Visible Fresnel diffraction from Hecht “Optics” I. K. Robinson, STRUBI Mar 2009 8 Diffuse Scattering acquires fine structure with a Coherent Beam I. K. Robinson, STRUBI Mar 2009 9 Coherent Diffraction from Crystals k Fourier Transform h I. K. Robinson, STRUBI Mar 2009 10 I. K. Robinson, STRUBI Mar 2009 11 Chemical Synthesis of Nanocrystals • Reactants introduced rapidly • High temperature solvent • Surfactant/organic capping agent • Square superlattice (200nm scale) C. -
Coherent Diffraction Imaging
Coherent Diffraction Imaging Ross Harder aka “The Imposter” Acknowledgments: Prof. Ian Robinson (BNL) 34-ID-C Dr. Xiaojing Huang (BNL) Advanced Photon Source Dr. Jesse Clark (PULSE institute, SLAC Amazon) Prof. Oleg Shpyrko (UCSD) Dr. Andrew Ulvestad (ANL – MSDTesla) https://tinyurl.com/y2qtrz7c Dr. Ian McNulty (ANL – CNM MaxIV) Dr. Junjing Deng (ANL – APS) Non-compact samples (Ptychography) Compact Objects (CDI) research papers implies an effective degree of transverse coherence at the oftenCOHERENCE associated with interference phenomena, where the source, as totally incoherent sources radiate into all directions mutual coherence function (MCF)1 (Goodman, 1985). À r ; r ; E à r ; t E r ; t 2 The transverse coherence area ÁxÁy of a synchrotron ð 1 2 Þ¼ ð 1 Þ ð 2 þ Þ ð Þ source can be estimated from Heisenberg’s uncertainty prin- plays the main role. It describes the correlations between two ciple (Mandel & Wolf, 1995), ÁxÁy h- 2=4Áp Áp , where x y complex values of the electric field E à r1; t and E r2; t at Áx; Áy and Áp ; Áp are the uncertainties in the position and ð Þ ð þ Þ x y different points r1 and r2 and different times t and t .The momentum in the horizontal and vertical direction, respec- brackets denote the time average. þ tively. Due to the de Broglie relation p = h- k,wherek =2=, Whenh weÁÁÁ consideri propagation of the correlation function the uncertainty in the momentum Áp can be associated with of the field in free space, it is convenient to introduce the the source divergence Á, Áp = h- kÁ,andthecoherencearea cross-spectral density function (CSD), W r1; r2; ! , which is in the source plane is given by defined as the Fourier transform of the MCFð (MandelÞ & Wolf, 1995), 2 1 ÁxÁy : 1 4 Á Á ð Þ W r1; r2; ! À r1; r2; exp i! d; 3 x y ð Þ¼ ð Þ ðÀ Þ ð Þ where ! Visibilityis the frequency of fringes is of a the direct radiation. -
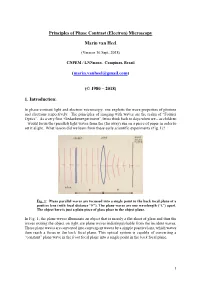
Principles of the Phase Contrast (Electron) Microscopy
Principles of Phase Contrast (Electron) Microscopy Marin van Heel (Version 16 Sept. 2018) CNPEM / LNNnano, Campinas, Brazil ([email protected]) (© 1980 – 2018) 1. Introduction: In phase-contrast light and electron microscopy, one exploits the wave properties of photons and electrons respectively. The principles of imaging with waves are the realm of “Fourier Optics”. As a very first “Gedankenexperiment”, let us think back to days when we – as children – would focus the (parallel) light waves from the (far away) sun on a piece of paper in order to set it alight. What lesson did we learn from these early scientific experiments (Fig. 1)? Fig. 1: Plane parallel waves are focussed into a single point in the back focal plane of a positive lens (with focal distance “F”). The plane waves are one wavelength (“λ”) apart. The object here is just a plain piece of glass place in the object plane. In Fig. 1, the plane waves illuminate an object that is merely a flat sheet of glass and thus the waves exiting the object on right are plane waves indistinguishable from the incident waves. These plane waves are converted into convergent waves by a simple positive lens, which waves then reach a focus in the back focal plane. This optical system is capable of converting a “constant” plane wave in the front focal plane into a single point in the back focal plane. 1 Fig. 2: A single point scatterer in the object plane leads to secondary (“scattered”) concentric waves emerging from that point in the object. Since the object is placed in the front focal plane, these scattered or “diffracted” secondary waves become plane waves hitting the back focal plane of the system. -

The Geometry of the Phase Retrieval Problem Charles L
The Geometry of the Phase Retrieval Problem Charles L. Epstein The Geometry of the Phase Retrieval Problem Introduction Operator Splitting Methods in Data Analysis Workshop Discrete Problem Torus Geometry Reconstruction Charles L. Epstein Algorithms Numerical Examples CCM at the Flatiron Institute University of Pennsylvania Other Conditions History of CDI March 20, 2019 Bibliography Acknowledgment The Geometry of the Phase Retrieval Problem Charles L. Epstein I want to thank Christian and Patrick for inviting me to speak. Introduction What I will discuss today is somewhat unconventional, ongoing Discrete Problem work, at the intersection of pure mathematics, numerical analysis Torus Geometry and mathematical physics. Reconstruction Algorithms This work was done jointly with Alex Barnett, Leslie Greengard, Numerical and Jeremy Magland and supported by the Centers for Examples Computational Biology and Mathematics at the Flatiron Institute Other Conditions of the Simons Foundation, New York, NY. History of CDI Bibliography Outline The Geometry of the Phase Retrieval Problem 1 Introduction Charles L. Epstein 2 The Discrete Phase Retrieval Problem Introduction 3 Geometry of the Torus Discrete Problem Torus Geometry 4 Reconstruction Algorithms Reconstruction Algorithms 5 Numerical Examples in Phase Retrieval Numerical Examples 6 Other Auxiliary Conditions Other Conditions History of CDI 7 Short History of CDI Bibliography 8 Bibliography High Resolution Imaging The Geometry of the Phase Retrieval Problem Charles L. Epstein Introduction Today I will speak about inverse problems that arise in Coherent Discrete Problem Diffraction Imaging. This is a technique that uses very high Torus Geometry energy, monochromatic light, either electrons or x-rays, to form Reconstruction high resolution images of animate and inanimate materials. -
Phase Problem in X-Ray Crystallography, and Its Solution Reciprocal Directions and Spacings from the Real Lattice
Phase Problem in X-ray Secondary article Crystallography, and Its Article Contents . The Phase Problem in X-ray Crystallography Solution . Patterson Methods . Direct Methods Kevin Cowtan, University of York, UK . Multiple Isomorphous Replacement . Multiwavelength Anomalous Dispersion (MAD) X-ray crystallography can provide detailed information about the structure of biological . Molecular Replacement molecules if the ‘phase problem’ can be solved for the molecule under study. The . Phase Improvement phase problem arises because it is only possible to measure the amplitude of diffraction . Summary spots: information on the phase of the diffracted radiation is missing. Techniques are available to reconstruct this information. The Phase Problem in X-ray called the unit cell. It is convenient to define crystal axes a, b Crystallography and c defining the unit cell in three dimensions. Scattering along directions reflecting the lattice repeat will be X-ray diffraction provides one of the most important tools reinforced by every repeat of the unit cell, and will be for examining the three-dimensional (3D) structure of strong; scattering along all other directions will be weak. biological macromolecules. The physics of diffraction As a result, the full diffraction pattern of the crystal is a requires that in order to resolve features of atomic pattern of spots, forming a three-dimensional lattice with structure it is necessary to employ radiation with a wavelength of the order of atomic spacing or smaller. X- rays have suitable wavelength, and interact with the Scattered waves are electrons of the atoms. However, the interaction between out of phase X-rays and electrons is weak, and such energetic radiation causes ionization of the constituent atoms of the molecule, damaging the molecule under study.