Dcas9-Targeted Locus-Specific Protein Isolation Method Identifies Histone Gene Regulators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production
The Journal of Neuroscience, March 25, 2015 • 35(12):4903–4916 • 4903 Development/Plasticity/Repair Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production Melody P. Lun,1,3 XMatthew B. Johnson,2 Kevin G. Broadbelt,1 Momoko Watanabe,4 Young-jin Kang,4 Kevin F. Chau,1 Mark W. Springel,1 Alexandra Malesz,1 Andre´ M.M. Sousa,5 XMihovil Pletikos,5 XTais Adelita,1,6 Monica L. Calicchio,1 Yong Zhang,7 Michael J. Holtzman,7 Hart G.W. Lidov,1 XNenad Sestan,5 Hanno Steen,1 XEdwin S. Monuki,4 and Maria K. Lehtinen1 1Department of Pathology, and 2Division of Genetics, Boston Children’s Hospital, Boston, Massachusetts 02115, 3Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, Massachusetts 02118, 4Department of Pathology and Laboratory Medicine, University of California Irvine School of Medicine, Irvine, California 92697, 5Department of Neurobiology and Kavli Institute for Neuroscience, Yale School of Medicine, New Haven, Connecticut 06510, 6Department of Biochemistry, Federal University of Sa˜o Paulo, Sa˜o Paulo 04039, Brazil, and 7Pulmonary and Critical Care Medicine, Department of Medicine, Washington University, St Louis, Missouri 63110 A sheet of choroid plexus epithelial cells extends into each cerebral ventricle and secretes signaling factors into the CSF. To evaluate whether differences in the CSF proteome across ventricles arise, in part, from regional differences in choroid plexus gene expression, we defined the transcriptome of lateral ventricle (telencephalic) versus fourth ventricle (hindbrain) choroid plexus. We find that positional identitiesofmouse,macaque,andhumanchoroidplexiderivefromgeneexpressiondomainsthatparalleltheiraxialtissuesoforigin.We thenshowthatmolecularheterogeneitybetweentelencephalicandhindbrainchoroidplexicontributestoregion-specific,age-dependent protein secretion in vitro. -

Synergistic Genetic Interactions Between Pkhd1 and Pkd1 Result in an ARPKD-Like Phenotype in Murine Models
BASIC RESEARCH www.jasn.org Synergistic Genetic Interactions between Pkhd1 and Pkd1 Result in an ARPKD-Like Phenotype in Murine Models Rory J. Olson,1 Katharina Hopp ,2 Harrison Wells,3 Jessica M. Smith,3 Jessica Furtado,1,4 Megan M. Constans,3 Diana L. Escobar,3 Aron M. Geurts,5 Vicente E. Torres,3 and Peter C. Harris 1,3 Due to the number of contributing authors, the affiliations are listed at the end of this article. ABSTRACT Background Autosomal recessive polycystic kidney disease (ARPKD) and autosomal dominant polycystic kidney disease (ADPKD) are genetically distinct, with ADPKD usually caused by the genes PKD1 or PKD2 (encoding polycystin-1 and polycystin-2, respectively) and ARPKD caused by PKHD1 (encoding fibrocys- tin/polyductin [FPC]). Primary cilia have been considered central to PKD pathogenesis due to protein localization and common cystic phenotypes in syndromic ciliopathies, but their relevance is questioned in the simple PKDs. ARPKD’s mild phenotype in murine models versus in humans has hampered investi- gating its pathogenesis. Methods To study the interaction between Pkhd1 and Pkd1, including dosage effects on the phenotype, we generated digenic mouse and rat models and characterized and compared digenic, monogenic, and wild-type phenotypes. Results The genetic interaction was synergistic in both species, with digenic animals exhibiting pheno- types of rapidly progressive PKD and early lethality resembling classic ARPKD. Genetic interaction be- tween Pkhd1 and Pkd1 depended on dosage in the digenic murine models, with no significant enhancement of the monogenic phenotype until a threshold of reduced expression at the second locus was breached. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Regulation of Neuronal Gene Expression and Survival by Basal NMDA Receptor Activity: a Role for Histone Deacetylase 4
The Journal of Neuroscience, November 12, 2014 • 34(46):15327–15339 • 15327 Cellular/Molecular Regulation of Neuronal Gene Expression and Survival by Basal NMDA Receptor Activity: A Role for Histone Deacetylase 4 Yelin Chen,1 Yuanyuan Wang,1 Zora Modrusan,3 Morgan Sheng,1 and Joshua S. Kaminker1,2 Departments of 1Neuroscience, 2Bioinformatics and Computational Biology, and 3Molecular Biology, Genentech Inc., South San Francisco, California 94080 Neuronal gene expression is modulated by activity via calcium-permeable receptors such as NMDA receptors (NMDARs). While gene expression changes downstream of evoked NMDAR activity have been well studied, much less is known about gene expression changes that occur under conditions of basal neuronal activity. In mouse dissociated hippocampal neuronal cultures, we found that a broad NMDAR antagonist, AP5, induced robust gene expression changes under basal activity, but subtype-specific antagonists did not. While some of the gene expression changes are also known to be downstream of stimulated NMDAR activity, others appear specific to basal NMDARactivity.ThegenesalteredbyAP5treatmentofbasalcultureswereenrichedforpathwaysrelatedtoclassIIahistonedeacetylases (HDACs), apoptosis, and synapse-related signaling. Specifically, AP5 altered the expression of all three class IIa HDACs that are highly expressed in the brain, HDAC4, HDAC5, and HDAC9, and also induced nuclear accumulation of HDAC4. HDAC4 knockdown abolished a subset of the gene expression changes induced by AP5, and led to neuronal death under -

Systems Biology Lecture Outline (Systems Biology) Michael K
Spring 2019 – Epigenetics and Systems Biology Lecture Outline (Systems Biology) Michael K. Skinner – Biol 476/576 CUE 418, 10:35-11:50 am, Tuesdays & Thursdays January 22 & 29, 2019 Weeks 3 and 4 Systems Biology (Components & Technology) Components (DNA, Expression, Cellular, Organ, Physiology, Organism, Differentiation, Development, Phenotype, Evolution) Technology (Genomics, Transcriptomes, Proteomics) (Interaction, Signaling, Metabolism) Omics (Data Processing and Resources) Required Reading ENCODE (2012) ENCODE Explained. Nature 489:52-55. Tavassoly I, Goldfarb J, Iyengar R. (2018) Essays Biochem. 62(4):487-500. Literature Sundaram V, Wang T. Transposable Element Mediated Innovation in Gene Regulatory Landscapes of Cells: Re-Visiting the "Gene-Battery" Model. Bioessays. 2018 40(1). doi: 10.1002/bies.201700155. Abil Z, Ellefson JW, Gollihar JD, Watkins E, Ellington AD. Compartmentalized partnered replication for the directed evolution of genetic parts and circuits. Nat Protoc. 2017 Dec;12(12):2493- 2512. Filipp FV. Crosstalk between epigenetics and metabolism-Yin and Yang of histone demethylases and methyltransferases in cancer. Brief Funct Genomics. 2017 Nov 1;16(6):320-325. Chen Z, Li S, Subramaniam S, Shyy JY, Chien S. Epigenetic Regulation: A New Frontier for Biomedical Engineers. Annu Rev Biomed Eng. 2017 Jun 21;19:195-219. Hollick JB. Paramutation and related phenomena in diverse species. Nat Rev Genet. 2017 Jan;18(1):5-23. Macovei A, Pagano A, Leonetti P, Carbonera D, Balestrazzi A, Araújo SS. Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: implications on seed technology traits. Plant Cell Rep. 2017 May;36(5):669-688. Bagchi DN, Iyer VR. -

The Human Proteome Co-Regulation Map Reveals Functional Relationships Between Proteins
bioRxiv preprint doi: https://doi.org/10.1101/582247; this version posted March 19, 2019. The copyright holder for this preprint (which was 19/03/2019not certified by peer review) is the author/funder, who hasNBT_manuscript_revised granted bioRxiv a license - toGoogle display Docs the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 The human proteome co-regulation map reveals functional relationships 2 between proteins 3 Georg Kustatscher1 *, Piotr Grabowski2 *, Tina A. Schrader3 , Josiah B. Passmore3 , Michael 4 Schrader3 , Juri Rappsilber1,2# 5 1 Wellcome Centre for Cell Biology, University of Edinburgh, Edinburgh EH9 3BF, UK 6 2 Bioanalytics, Institute of Biotechnology, Technische Universität Berlin, 13355 Berlin, 7 Germany 8 3 Biosciences, University of Exeter, Exeter EX4 4QD, UK 9 * Equal contribution 10 # Communicating author: [email protected] 11 Submission as Resource article. 12 The annotation of protein function is a longstanding challenge of cell biology that 13 suffers from the sheer magnitude of the task. Here we present ProteomeHD, which 14 documents the response of 10,323 human proteins to 294 biological perturbations, 15 measured by isotope-labelling mass spectrometry. Using this data matrix and robust 16 machine learning we create a co-regulation map of the cell that reflects functional 17 associations between human proteins. The map identifies a functional context for 18 many uncharacterized proteins, including microproteins that are difficult to study with 19 traditional methods. Co-regulation also captures relationships between proteins 20 which do not physically interact or co-localize. For example, co-regulation of the 21 peroxisomal membrane protein PEX11β with mitochondrial respiration factors led us 22 to discover a novel organelle interface between peroxisomes and mitochondria in 23 mammalian cells. -
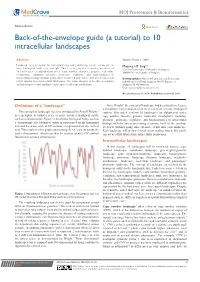
Back-Of-The-Envelope Guide (A Tutorial) to 10 Intracellular Landscapes
MOJ Proteomics & Bioinformatics Review Article Open Access Back-of-the-envelope guide (a tutorial) to 10 intracellular landscapes Abstract Volume 7 Issue 1 - 2018 Landscape is a metaphor for conceptualizing and visualizing a score across one or Maurice HT Ling1,2 more biological entities or concepts. This review provides a cursory overview of 1Colossus Technologies LLP, Republic of Singapore 10 landscapes (in alphabetical order, copy number, fluxome, genome, molecular, 2HOHY Pte. Ltd., Republic of Singapore metabolome, mutation, phenome, proteome, regulome, and transcriptome) in intracellular biology without going into extensive depth; hence, this article can act as Correspondence: Maurice HT Ling, Colossus Technologies a first tutorial into intracellular landscapes. The value ahead is to be able to compare LLP, 8 Burns Road, Trivex, Singapore 369977, Republic of and interrogate across multiple landscapes at different resolutions. Singapore, Tel +6596669233, Email [email protected] Received: January 29, 2018 | Published: February 06, 2018 Definition of a “landscape” Since Wright,1 the concept of landscape had been used to reference a metaphoric representation of an overview of one or more biological 1 The concept of landscape was first introduced by Sewell Wright, entities. This article reviews 10 landscapes (in alphabetical order, as a metaphor to visual a scale or score across a biological entity, copy number, fluxome, genome, molecular, metabolome, mutation, such as a chromosome (Figure 1). In a linear biological entity, such as phenome, proteome, regulome, and transcriptome) in intracellular a chromosome; the biological entity is represented on the horizontal biology with the aim of presenting a cursory, back of the envelope axis and the score, such as GC content, is represented on the vertical overview without going into extensive depth into each landscape. -

Bioinformatics Strategies for Identification of Cancer Biomarkers and Targets in Pathogens Associated with Cancer
FEDERAL UNIVERSITY OF MINAS GERAIS INSTITUTE OF BIOLOGICAL SCIENCE DEPARTMENT OF GENERAL BIOLOGY GRADUATE PROGRAM OF BIOINFORMATICS BIOINFORMATICS STRATEGIES FOR IDENTIFICATION OF CANCER BIOMARKERS AND TARGETS IN PATHOGENS ASSOCIATED WITH CANCER PhD STUDENT: Debmalya Barh SUPERVISOR: Prof. Dr. Vasco Ariston de Carvalho Azevedo Page 1 of 237 DEBMALYA BARH Bioinformatics strategies for identification of cancer biomarkers and targets in pathogens associated with cancer PhD STUDENT: Debmalya Barh SUPERVISOR: Prof. Dr. Vasco Ariston de Carvalho Azevedo FEDERAL UNIVERSITY OF MINAS GERAIS INSTITUTE OF BIOLOGICAL SCIENCES BELO HORIZONTE - MG February – 2017 Page 2 of 237 043 Barh, Debmalya. Bioinformatics strategies for identification of cancer biomarkers and targets in pathogens associated with cancer [manuscrito] / Debmalya Barh. – 2017. 237 f. : il. ; 29,5 cm. Supervisor: Vasco Ariston de Carvalho Azevedo. Tese (doutorado) – Federal University of Minas Gerais, Institute of Biological Sciences. 1. Bioinformática - Teses. 2. Marcadores biológicos. 3. Doenças transmissíveis - Teses. 4. Transcriptômica - Teses. 5. MicroRNAs - Teses. I. Azevedo , Vasco Ariston de Carvalho. II. Universidade Federal de Minas Gerais. Instituto de Ciências Biológicas. III. Título. CDU: 573:004 TABLE OF CONTENTS TABLE OF CONTENTS……………………………………………………………………………………i DEDICATION………………………………………………………………………………………..……iii ACKNOWLEDGEMENTS…………………………………………………………………………..……iv LIST OF ABBREVIATIONS…………………………………………………………………….……….. v ABSTRACT………………………………………………………………………………………...……..vii -

Mrna Structure Dynamics Identifies the Embryonic RNA Regulome
bioRxiv preprint doi: https://doi.org/10.1101/274290; this version posted March 1, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. mRNA structure dynamics identifies the embryonic RNA regulome Jean-Denis Beaudoin1,*,‡, Eva Maria Novoa2,3,4,5,*, Charles E Vejnar1, Valeria Yartseva1, Carter Takacs1, Manolis Kellis2,3 and Antonio J Giraldez1,6,‡ RNA folding plays a crucial role in RNA function. However, our knowledge of the global structure of the transcriptome is limited to steady-state conditions, hindering our understanding of how RNA structure dynamics influences gene function. Here, we have characterized mRNA structure dynamics during zebrafish development. We observe that on a global level, translation guides structure rather than structure guiding translation. We detect a decrease in structure in translated regions, and we identify the ribosome as a major remodeler of RNA structure in vivo. In contrast, we find that 3’-UTRs form highly folded structures in vivo, which can affect gene expression by modulating miRNA activity. Furthermore, we find that dynamic 3’-UTR structures encode RNA decay elements, including regulatory elements in nanog and cyclin A1, key maternal factors orchestrating the maternal-to-zygotic transition. These results reveal a central role of RNA structure dynamics in gene regulatory programs. Introduction Previous studies have focused on the global analysis of RNA structures in cells at RNA carries out a broad range of functions,1 and steady-state. However, in an organism, critical RNA structure has emerged as a fundamental developmental and metabolic decisions are often regulatory mechanism, modulating various post- made when cells transition between cellular transcriptional events such as splicing2, states. -

Organizational Properties of a Functional Mammalian Cis-Regulome
bioRxiv preprint doi: https://doi.org/10.1101/550897; this version posted February 15, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Organizational properties of a functional mammalian cis-regulome Virendra K. Chaudhri, Krista Dienger-Stambaugh, Zhiguo Wu, Mahesh Shrestha and Harinder Singh*# Division of Immunobiology and Center for Systems Immunology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, 45220, USA #Current address: Center for Systems Immunology, Departments of Immunology and Computational and Systems Biology, The University of Pittsburgh, Pittsburgh, PA 15213 *Corresponding author Email: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/550897; this version posted February 15, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Mammalian genomic states are distinguished by their chromatin and transcription profiles. Most genomic analyses rely on chromatin profiling to infer cis-regulomes controlling distinctive cellular states. By coupling FAIRE-seq with STARR-seq and integrating Hi-C we assemble a functional cis-regulome for activated murine B- cells. Within 55,130 accessible chromatin regions we delineate 9,989 active enhancers communicating with 7,530 promoters. The cis-regulome is dominated by long range enhancer-promoter interactions (>100kb) and complex combinatorics, implying rapid evolvability. Genes with multiple enhancers display higher rates of transcription and multi-genic enhancers manifest graded levels of H3K4me1 and H3K27ac in poised and activated states, respectively. Motif analysis of pathway-specific enhancers reveals diverse transcription factor (TF) codes controlling discrete processes. -

Comparative Gene Expression Profiles in Parathyroid Adenoma
Journal of Clinical Medicine Article Comparative Gene Expression Profiles in Parathyroid Adenoma and Normal Parathyroid Tissue Young Jun Chai 1,† , Heejoon Chae 2,† , Kwangsoo Kim 3 , Heonyi Lee 2, Seongmin Choi 3 , Kyu Eun Lee 4 and Sang Wan Kim 5,* 1 Department of Surgery, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul 07061, Korea; [email protected] 2 Division of Computer Science, Sookmyung Women’s University, Seoul 04310, Korea; [email protected] (H.C.); [email protected] (H.L.) 3 Division of Clinical Bioinformatics, Biomedical Research Institute, Seoul National University Hospital, Seoul 03080, Korea; [email protected] (K.K.); [email protected] (S.C.) 4 Department of Surgery, Seoul National University Hospital & College of Medicine, Seoul 03080, Korea; [email protected] 5 Department of Internal Medicine, Seoul National University College of Medicine, and Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul 07061, Korea * Correspondence: [email protected]; Tel.: +82-2-870-2223 † These two authors contributed equally. Received: 29 January 2019; Accepted: 22 February 2019; Published: 2 March 2019 Abstract: Parathyroid adenoma is the main cause of primary hyperparathyroidism, which is characterized by enlarged parathyroid glands and excessive parathyroid hormone secretion. Here, we performed transcriptome analysis, comparing parathyroid adenomas with normal parathyroid gland tissue. RNA extracted from ten parathyroid adenoma and five normal parathyroid samples was sequenced, and differentially expressed genes (DEGs) were identified using strict cut-off criteria. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using DEGs as the input, and protein-protein interaction (PPI) networks were constructed using Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) and visualized in Cytoscape. -

The Genetic Program of Pancreatic Beta-Cell Replication in Vivo
Page 1 of 65 Diabetes The genetic program of pancreatic beta-cell replication in vivo Agnes Klochendler1, Inbal Caspi2, Noa Corem1, Maya Moran3, Oriel Friedlich1, Sharona Elgavish4, Yuval Nevo4, Aharon Helman1, Benjamin Glaser5, Amir Eden3, Shalev Itzkovitz2, Yuval Dor1,* 1Department of Developmental Biology and Cancer Research, The Institute for Medical Research Israel-Canada, The Hebrew University-Hadassah Medical School, Jerusalem 91120, Israel 2Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel. 3Department of Cell and Developmental Biology, The Silberman Institute of Life Sciences, The Hebrew University of Jerusalem, Jerusalem 91904, Israel 4Info-CORE, Bioinformatics Unit of the I-CORE Computation Center, The Hebrew University and Hadassah, The Institute for Medical Research Israel- Canada, The Hebrew University-Hadassah Medical School, Jerusalem 91120, Israel 5Endocrinology and Metabolism Service, Department of Internal Medicine, Hadassah-Hebrew University Medical Center, Jerusalem 91120, Israel *Correspondence: [email protected] Running title: The genetic program of pancreatic β-cell replication 1 Diabetes Publish Ahead of Print, published online March 18, 2016 Diabetes Page 2 of 65 Abstract The molecular program underlying infrequent replication of pancreatic beta- cells remains largely inaccessible. Using transgenic mice expressing GFP in cycling cells we sorted live, replicating beta-cells and determined their transcriptome. Replicating beta-cells upregulate hundreds of proliferation- related genes, along with many novel putative cell cycle components. Strikingly, genes involved in beta-cell functions, namely glucose sensing and insulin secretion were repressed. Further studies using single molecule RNA in situ hybridization revealed that in fact, replicating beta-cells double the amount of RNA for most genes, but this upregulation excludes genes involved in beta-cell function.