December 2008 KEY to EVIDENCE STATEMENTS and GRADES of RECOMMENDATIONS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification of Pressures and Impacts Arising Frm Strategic Development
Report for Scottish Environment Protection Agency/ Neil Deasley Planning and European Affairs Manager Scottish Natural Heritage Scottish Environment Protection Agency Erskine Court The Castle Business Park Identification of Pressures and Impacts Stirling FK9 4TR Arising From Strategic Development Proposed in National Planning Policy Main Contributors and Development Plans Andrew Smith John Pomfret Geoff Bodley Neil Thurston Final Report Anna Cohen Paul Salmon March 2004 Kate Grimsditch Entec UK Limited Issued by ……………………………………………… Andrew Smith Approved by ……………………………………………… John Pomfret Entec UK Limited 6/7 Newton Terrace Glasgow G3 7PJ Scotland Tel: +44 (0) 141 222 1200 Fax: +44 (0) 141 222 1210 Certificate No. FS 13881 Certificate No. EMS 69090 09330 h:\common\environmental current projects\09330 - sepa strategic planning study\c000\final report.doc In accordance with an environmentally responsible approach, this document is printed on recycled paper produced from 100% post-consumer waste or TCF (totally chlorine free) paper COMMISSIONED REPORT Summary Report No: Contractor : Entec UK Ltd BACKGROUND The work was commissioned jointly by SEPA and SNH. The project sought to identify potential pressures and impacts on Scottish Water bodies as a consequence of land use proposals within the current suite of Scottish development Plans and other published strategy documents. The report forms part of the background information being collected by SEPA for the River Basin Characterisation Report in relation to the Water Framework Directive. The project will assist SNH’s environmental audit work by providing an overview of trends in strategic development across Scotland. MAIN FINDINGS Development plans post 1998 were reviewed to ensure up-to-date and relevant information. -

203 Local Police & Fire Scrutiny Committee – 6 June
203 LOCAL POLICE & FIRE SCRUTINY COMMITTEE – 6 JUNE 2019 _______________________________________________________________________ Local Police & Fire Scrutiny Committee Thursday 6 June 2019 at 2pm Present: Councillors Clocherty, Crowther, Curley, Jackson, J McEleny, McVey, Murphy and Quinn. Chair: Councillor McVey presided. In attendance: Corporate Director Education, Communities & Organisational Development, Head of Culture, Communities & Educational Resources, Service Manager, Community Learning & Development, Community Safety & Resilience and Sport, Mr I Hanley (Community Safety & Resilience), Service Manager, Public Protection, Mr J Douglas (for Head of Legal & Property Services) and Ms S Lang (Legal & Property Services). In attendance also: Detective Superintendent P Livingstone (for Chief Superintendent G Crossan) and Sergeant J Logsdon, Police Scotland, Area Manager G Binning and Group Manager D McCarrey, Scottish Fire & Rescue Service. The following paragraphs are submitted for information only, having been dealt with under the powers delegated to the Committee. Prior to the commencement business, the Convener referred to the forthcoming retiral of Chief Superintendent Gordon Crossan and, on behalf of the Committee, he asked that his appreciation be extended to Mr Crossan for his 30 years’ Police service and, in particular, for his two years’ service as Divisional Commander for K Division. 401 Apologies, Substitutions and Declarations of Interest 401 Apologies for absence were intimated on behalf of Councillors MacLeod, Moran and Wilson. No declarations of interest were intimated. 402 Scottish Fire and Rescue Service – Spotlight on Deliberate Fires in Inverclyde: 1 402 April 2018 – 31 March 2019 There was submitted a report by the Scottish Fire & Rescue Service providing details of deliberate fire incidents which the service attended within Inverclyde during the period 1 April 2018 to 31 March 2019. -
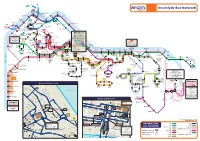
Inverclyde Bus Network
Fe rry to K D i F l un c e o r r o e n r y g g t o a n Inverclyde Bus Network 540 Gourock Pierhead Eldon Street Shore Street, Albert Road Battery 547 Ferry Terminal Park 901 Cardwell Ashton Bay 907 Road 507 Midton Fort Hunter’s Tower Drive Drumshantie Matilda Brougham Street Quay Road 507 Dunoon 547 Divert Road 507 Reservoir Road 907 Fe rr y to Dunoon McInroy’s Point Union Street Ferry Terminal 538 Kirn Drive Mallard Crescent Earnhill Lyle Road Greenock Levan 507 Road 535 Hilltop Cloch Road Trumpethill Road Bus Station, Finch Grieve Road Kilblain Street/ Services Road that commence Weymouth Nelson West Stewart St. Depot 545H at Earnhill Road: Crescent Street 507 517 531 517,547 535 545 545 545 901,906,907 532.533 538 Calling: Inverclyde 545H Wren Tasker Street 531,532,533, Royal Road 543 545H 547 Rue End 901,906,907,X22 Cardwell Hospital Bow Road Garden Banff 550 576.578 X22 Street Port Centre Road Calling: 531,532,533 535,540,545, 901,906,907 Main Glasgow Greenock 517 Fancy Farm Road 538 Bridgend River Clyde Larkfield 901,906,907 X22 Street Lilybank Bus Station Road 901,906,907,X22 Burns Square 517,535,540 Bishopton Cumberland 576.578,906 Glasgow 901 517 Inverkip Street Sir Michael Road Street Bogston Road Inverkip 550 Regent 540 Arthur Ratho Ladyburn Woodhall Bishopton Road Street Street Greenock Street Erskine Hospital 543 533,535 Broadfield Kilpatrick IBM Branchton Ravenscraig 550 Health Centre Golden Jubilee Hospital 535 Gibshill 532 Clune Brae Parkhill X22 National Hospital Cartsdyke Avenue Baker 543 Belville River Clyde Roxburgh -

Review Decision Notice
Inverclyde Local Review Body Our Ref: 12/0220/IC REVIEW DECISION NOTICE Decision by Inverclyde Local Review Body (the ILRB) Site address: Gibshill Road, Greenock Application for Review by Canata & Seggie, Chartered Architects on behalf of Chris Wright & Sons Limited against the decision by an appointed officer of Inverclyde Council Application Ref: 12/0220/IC Application Drawings: Drawing No. 2165 - D - 001 Revision A Existing Site Layout Plan Drawing No. 2165 - D - 002 Revision A Proposed Site Layout Plan Date of Review Decision Notice: 22 March 2013 Decision The ILRB upholds the decision to refuse planning permission for the reasons given below and dismisses the review. Attention is also drawn to the Advisory Notice at the end of this Review Decision Notice. 1. Introduction 1.1 This Notice constitutes the formal decision notice of the ILRB as required by the Town and Country Planning (Schemes of Delegation and Local Review Procedure) (Scotland) Regulations 2008. 1.2 The above application for planning permission was considered by the ILRB at a meeting held on 6 March 2013. The ILRB was constituted by Provost R Moran, Councillors G Dorrian, T Loughran and D Wilson (Chair). 2. Proposal 2.1 The application proposal is for planning permission to form a vehicular access onto Gibshill Road and spread demolition material, which has been imported to the site without the benefit of planning permission, to an approximate depth of 500 mm across the site. Thereafter it is proposed to bring the site into use as a demolition contractor's storage and distribution yard. The use of the yard is to be limited to the storage of plant and vehicles, with no storage or crushing of demolition material. -

The Cloch Book 2018 (Low Res
It gives me great pleasure to introduce the Cloch Souvenir Book celebrating 50 years of Cloch. This is our story of growth and development in the Inverclyde area since 1968. This book has been entirely funded from the Heritage Lottery Fund and the end result is a humorous, informative and interactive journey through the decades, providing readers with clips from our history so far. Among our contributors are our tenants, our staff and board members and all made possible with the artistic flair of the Magic Torch from Greenock. I hope that readers will enjoy the book even half as much as we enjoyed making it. Inverclyde has a really proud history and Cloch is a key part of that and will be for many years to come. Paul McVey, Director Magic Torch Comics would like to thank everyone who took the If you spot a QR code time to help out and share their stories with us. Every story in the book, scan it you will read is based on true events - though we did change a on your phone with a few of the names here and there. Special thanks from us to all QR reader for bonus of the Cloch Housing team, but especially Mick McKendrick and content. Scan here to Liz Bowden whose imagination, enthusiasm and patience has see Cloch’s special helped make the project work. 50th anniversary film Artwork by Andy Lee (p5,6,8,9,11,12,21,24,27-29) Curt-S (p15,22) Mhairi Robertson (p13) Clochie’s Race Through Time by William Rice & Black Cassidy www.magictorchcomics.co.uk / @magictorchcomix Cloch Housing Association OPENING TIMES 19 Bogle Street Monday 9:00AM - 5:00PM PA15 1ER, Greenock Tuesday 9:00AM - 5:00PM Phone: 01475 783637 Wednesday 9:00AM - 5:00PM Thursday 9:00AM - 6:00PM Inverclyde Care & Repair Friday 9:00AM - 4:00PM 19 Bogle Street PA15 1ER, Greenock Phone: 01475 78827 Registered Scottish Charity No. -

Newspaper Index H
Watt Library, Greenock Newspaper Index This index covers stories that have appeared in newspapers in the Greenock, Gourock and Port Glasgow area from the start of the nineteenth century. It is provided to researchers as a reference resource to aid the searching of these historic publications which can be consulted, preferably by prior appointment, at the Watt Library, 9 Union Street, Greenock. Subject Entry Newspaper Date Page H.D. Lee Ltd., Greenock H D Lee Ltd, clothing manufacturers, opening another factory on the Larkfield Estate Greenock Telegraph 22/01/1972 1 creating 500 new jobs H.D. Lee Ltd., Greenock Opening of clothing factory of H D Lee, Inc. Kansas on Larkfield Industrial Estate Greenock Telegraph 13/06/1970 7 Hairdressers' Society General meeting to discuss proposal to dissolve society. Greenock Advertiser 09/08/1831 3 Hairdressers' Society Dissolved 20 January, payment to all members and widows of members. Greenock Advertiser 19/02/1835 3 Haltonridge Farm, Kilmacolm Haltonridge Farm to be let Greenock Advertiser 11/05/1812 1 Hamburg Article on Hamburg Amerika and Norddeutscher Lloyd Lines which not only used the Greenock Telegraph 28/07/1983 16 Amerika/Norddeutscher Lloyd harbours but had ships built in Greenock. Lines Hamburg-Amerika Line Article on Hamburg-Amerika and Norddeutscher Lloyds line which used local harbours Greenock Telegraph 28/07/1983 16 Hamilton Free Church, Port Brief history of Hamilton Free Church and description Greenock Telegraph 09/06/1894 2 Glasgow Hamilton Free Church, Port Centenary of Hamilton -

Handbook 2019-20
Gibshill Children’s Centre Handbook 2019-20 Welcome to Gibshill Children’s Centre Gibshill Children’s Centre has been on this site in Gibshill since the 1970’s. It was initially an Urban Aid Funded Project and was then taken over by Education Services. In 2000, the Centre was substantially upgraded and we now have a beautiful, bright, airy building. The Centre provides a high standard of care and education which we hope that you will become aware of when you visit. We believe that close links between Home and Centre are essential and that working in partnership with you will provide the best service for you and your child. We believe in nurturing an environment where children and adults will feel safe, secure and happy. We will value their individuality, abilities and skills and ensure that their achievements are celebrated. This handbook gives you information about the Centre, however if there is any further information you require please do not hesitate to approach myself or any member of staff. We look forward to working alongside you in providing a happy, exciting and challenging experience for your child when attending our Centre. Janine Burns Head of Centre CONTENTS Page Aims and values Staff Session times, Age range of children, Centre Security, Car Park, Fund Money Admissions, Transition, Settling in period, Wrapround Service Term time holidays Our Curriculum & Transitions The four Capacities Literacy & English Mathematics Science Expressive Arts Health & Wellbeing Religions & Moral Studies Social Studies Technologies Special -

Proposed Development Site Assessment
Introduction This document sets out an initial assessment of sites that have been suggested to the Council as suitable for development through the 2020 call for sites process, and through internal identification. It sets out the factors the Council has taken into account in reaching its preferred position on the suggested sites as set out in the Main Issues Report. It will be updated to reflect comments and further submissions received during the Main Issues Report consultation. 1 Sites submitted for inclusion within Local Development Plan Ref Site Town/village Page CFS01 Carsemeadow Quarrier’s Village 4 CFS02 Kaimes Grove Quarrier’s Village 11 CFS03 North Denniston Kilmacolm 14 CFS04 Knapps 1 Kilmacolm 20 CFS05 Knapps 2 Kilmacolm 25 CFS06 Police Station Field Kilmacolm 30 CFS07 Lochwinnoch Road Kilmacolm 36 CFS08 Land at Overton, West Glen Road Kilmacolm 41 CFS09 Smithy Brae Kilmacolm 46 CFS10 Port Glasgow Road (1) Kilmacolm 50 CFS11 Port Glasgow Road (2) Kilmacolm 54 CFS12 Knockbuckle Road Kilmacolm 58 CFS13 Quarry Drive Kilmacolm 62 CFS14 Migdale Kilmacolm 67 CFS15 Planetreeyetts Kilmacolm 71 CFS16 Stables Wood Kilmacolm 76 CFS17 Arran Avenue Port Glasgow * CFS18 Port Glasgow Industrial Estate – South Port Glasgow 80 CFS19 Port Glasgow Industrial Estate Port Glasgow 84 CFS20 Port Glasgow Industrial Estate - North Port Glasgow 88 CFS21 Port Glasgow Industrial Estate - West Port Glasgow 92 CFS22 Barrs Brae (south) Port Glasgow 96 CFS23 Barrs Brae (north) Port Glasgow 100 CFS24 Bay Street Port Glasgow 104 CFS25 Gibshill Road Greenock 108 -

Inverclyde Green Network Study
Inverclyde Green Network Study Prepared by Land Use Consultants for Inverclyde Council, Riverside Inverclyde, Communities Scotland and The GCV Green Network Partnership December 2008 Inverclyde Green Network Study Prepared for Inverclyde Council, Riverside Inverclyde, Communities Scotland and Glasgow Clyde Valley Green Network Partnership by Land Use Consultants December 2008 37 Otago Street Glasgow G12 8JJ Tel: 0141 334 9595 Fax: 0141 334 7789 [email protected] CONTENTS Executive Summary.................................................................................... 1. Introduction ......................................................................................... 1 Inverclyde Today.........................................................................................................................................1 New Developments....................................................................................................................................3 Project Aims.................................................................................................................................................4 Methodology................................................................................................................................................................. 5 Structure of the Report.............................................................................................................................5 2. The Importance of the Green network ........................................... -

Promoting Excellence and Equity for All
PROMOTING EXCELLENCE AND EQUITY FOR ALL 2017 www.education.gov.scot Exhibition Partner WELCOME TO THE SCOTTISH LEARNING FESTIVAL 2017 The Scottish Learning Festival, Scotland’s key educational event, is taking place on 20 and 21 HOW TO BOOK YOUR PLACE September in the SEC, Glasgow. SLF is completely FREE for everyone to Over the years we’ve welcomed more than attend and contributes towards your career- 55,000 thousand educational practitioners from long professional learning. Scotland and beyond, and we look forward to To book your place, browse the conference welcoming thousands more this year. programme, note the seminars you want to attend and visit the Education Scotland The unique SLF experience will provide website education.gov.scot, for booking opportunities to access high-quality and highly information. Remember to book early to engaging professional learning as well as guarantee a place at the sessions of your opportunities to explore, discuss and exchange choice. knowledge and ideas with thousands of professional colleagues and experts. You are able to book the following each day: SLF 2017 has been planned around the key themes of promoting excellence and equity for Wednesday 20 September – opening all through: keynote address and three other sessions Thursday 21 September – opening keynote • empowering teachers, practitioners, parents, address and three other sessions schools and communities Attendance at the opening keynotes must • strengthening partnerships, collaboration be pre-booked as places are limited and are and networks allocated on a first-come, first-served basis. • building the professional capacity of Extra availability on the day teachers, practitioners and leaders While you are limited to eight choices • fair and learner-centred funding through the pre-booking system, you will be able to attend additional seminars on the • responsibility and accountability at all levels. -

Business Bulletin Iris Ghnothaichean
Monday 7 January 2019 Business Bulletin Iris Ghnothaichean Today's Business Meeting of the Parliament Committee Meetings There are no meetings today. There are no meetings today. Monday 7 January 2019 1 Today's Business Future Business Motions & Questions Legislation Other Gnothaichean an-diugh Gnothaichean ri teachd Gluasadan agus Ceistean Reachdas Eile Chamber | Seòmar Meeting of the Parliament There are no meetings today. Monday 7 January 2019 2 Today's Business Future Business Motions & Questions Legislation Other Gnothaichean an-diugh Gnothaichean ri teachd Gluasadan agus Ceistean Reachdas Eile Committees | Comataidhean Committee Meetings There are no meetings today. Monday 7 January 2019 3 Today's Business Future Business Motions & Questions Legislation Other Gnothaichean an-diugh Gnothaichean ri teachd Gluasadan agus Ceistean Reachdas Eile Chamber | Seòmar Future Meetings of the Parliament Business Programme agreed by the Parliament on 19 December 2018 Tuesday 8 January 2019 2:00 pm Time for Reflection: Reverend Colin Sinclair, Minister, Palmerston Place Church, Edinburgh followed by Parliamentary Bureau Motions followed by Topical Questions (if selected) followed by Scottish Government Debate: Ultra Low Emission Vehicles followed by Committee Announcements followed by Business Motions followed by Parliamentary Bureau Motions 5:00 pm Decision Time followed by Members' Business — S5M-14266 Brian Whittle: Transport Infrastructure in South West Scotland Wednesday 9 January 2019 2:00 pm Parliamentary Bureau Motions 2:00 pm Portfolio -

Ref: SL/AI Date: 27 February 2020 a Meeting of the Education
Municipal Buildings, Greenock PA15 1LY Ref: SL/AI Date: 27 February 2020 A meeting of the Education & Communities Committee will be held on Tuesday 10 March 2020 at 2pm within the Municipal Buildings, Greenock. Please note that consideration of the Education items of business will commence at 4pm or following conclusion of the Communities business, whichever is the later. GERARD MALONE Head of Legal and Property Services BUSINESS 1. Apologies, Substitutions and Declarations of Interest Page COMMUNITIES PERFORMANCE MANAGEMENT 2. Communities 2019/20 Revenue Budget - Period 9 to 31 December 2019 Report by Chief Financial Officer and Corporate Director Education, Communities & p Organisational Development 3. Communities Capital Programme 2019-2023 Progress Report by Corporate Director Education, Communities & Organisational p Development and Chief Financial Officer NEW BUSINESS 4. Inverclyde Anti-Social Behaviour Strategy 2020-2025 Report by Corporate Director Education, Communities & Organisational p Development 5. Pilot on the Provision of Crawl Spaces on Secondary School 3G Pitches Report by Corporate Director Education, Communities & Organisational p Development ITEMS FOR NOTING 6. Items for Noting (Communities) Report by Corporate Director Environment, Regeneration & Resources p 00 Agenda E&C - 10 03 2020 6(a) Extension to the Opening of Gourock Outdoor Pool Report by Corporate Director Education, Communities & Organisational p Development 6(b) Inverclyde Heritage Strategy 2019-29 Report by Corporate Director Education, Communities & Organisational p Development 6(c) Grants to Voluntary Organisations 2019/20 – Round 2 Report by Corporate Director Education, Communities & Organisational p Development 6(d) Under 19s Sports Grants 2019/20 – Round 2 Report by Corporate Director Education, Communities & Organisational p Development EDUCATION PERFORMANCE MANAGEMENT 7.