Bioinformatics Analysis of Differentially Expressed Proteins in Alcoholic Fatty Liver Disease Treated with Recombinant Human Cytoglobin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gene Essentiality Landscape and Druggable Oncogenic Dependencies in Herpesviral Primary Effusion Lymphoma
ARTICLE DOI: 10.1038/s41467-018-05506-9 OPEN Gene essentiality landscape and druggable oncogenic dependencies in herpesviral primary effusion lymphoma Mark Manzano1, Ajinkya Patil1, Alexander Waldrop2, Sandeep S. Dave2, Amir Behdad3 & Eva Gottwein1 Primary effusion lymphoma (PEL) is caused by Kaposi’s sarcoma-associated herpesvirus. Our understanding of PEL is poor and therefore treatment strategies are lacking. To address this 1234567890():,; need, we conducted genome-wide CRISPR/Cas9 knockout screens in eight PEL cell lines. Integration with data from unrelated cancers identifies 210 genes as PEL-specific oncogenic dependencies. Genetic requirements of PEL cell lines are largely independent of Epstein-Barr virus co-infection. Genes of the NF-κB pathway are individually non-essential. Instead, we demonstrate requirements for IRF4 and MDM2. PEL cell lines depend on cellular cyclin D2 and c-FLIP despite expression of viral homologs. Moreover, PEL cell lines are addicted to high levels of MCL1 expression, which are also evident in PEL tumors. Strong dependencies on cyclin D2 and MCL1 render PEL cell lines highly sensitive to palbociclib and S63845. In summary, this work comprehensively identifies genetic dependencies in PEL cell lines and identifies novel strategies for therapeutic intervention. 1 Department of Microbiology-Immunology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA. 2 Duke Cancer Institute and Center for Genomic and Computational Biology, Duke University, Durham, NC 27708, USA. 3 Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA. Correspondence and requests for materials should be addressed to E.G. (email: [email protected]) NATURE COMMUNICATIONS | (2018) 9:3263 | DOI: 10.1038/s41467-018-05506-9 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-018-05506-9 he human oncogenic γ-herpesvirus Kaposi’s sarcoma- (IRF4), a critical oncogene in multiple myeloma33. -

Large-Scale Image-Based Profiling of Single-Cell Phenotypes in Arrayed CRISPR-Cas9 Gene Perturbation Screens
Published online: January 23, 2018 Method Large-scale image-based profiling of single-cell phenotypes in arrayed CRISPR-Cas9 gene perturbation screens Reinoud de Groot1 , Joel Lüthi1,2 , Helen Lindsay1 , René Holtackers1 & Lucas Pelkmans1,* Abstract this reason, CRISPR-Cas9 has been used in large-scale functional genomic screens (Shalem et al, 2015). Most screens performed to High-content imaging using automated microscopy and computer date employ a pooled screening strategy, which can identify genes vision allows multivariate profiling of single-cell phenotypes. Here, that cause differential growth in screening conditions (Koike-Yusa we present methods for the application of the CISPR-Cas9 system et al, 2013; Shalem et al, 2014; Wang et al, 2014). However, in large-scale, image-based, gene perturbation experiments. We pooled screening precludes multivariate profiling of single-cell show that CRISPR-Cas9-mediated gene perturbation can be phenotypes. This can be partially overcome by combining pooled achieved in human tissue culture cells in a timeframe that is screening with single-cell RNA-seq, but this does not easily scale compatible with image-based phenotyping. We developed a pipe- to the profiling of thousands of single cells from thousands of line to construct a large-scale arrayed library of 2,281 sequence- perturbations, and is limited to features that can be read from verified CRISPR-Cas9 targeting plasmids and profiled this library RNA transcript profiles (Adamson et al, 2016; Dixit et al, 2016; for genes affecting cellular morphology and the subcellular local- Jaitin et al, 2016; Datlinger et al, 2017). Moreover, sequencing- ization of components of the nuclear pore complex (NPC). -

Comparative Proteomics Analysis of Human Liver Microsomes and S9
DMD Fast Forward. Published on November 7, 2019 as DOI: 10.1124/dmd.119.089235 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 89235 Comparative Proteomics Analysis of Human Liver Microsomes and S9 Fractions Xinwen Wang, Bing He, Jian Shi, Qian Li, and Hao-Jie Zhu Department of Clinical Pharmacy, University of Michigan, Ann Arbor, Michigan (X.W., B.H., J.S., H.-J.Z.); and School of Life Science and Technology, China Pharmaceutical University, Nanjing, Jiangsu, 210009 (Q.L.) Downloaded from dmd.aspetjournals.org at ASPET Journals on October 2, 2021 1 DMD Fast Forward. Published on November 7, 2019 as DOI: 10.1124/dmd.119.089235 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 89235 Running title: Comparative Proteomics of Human Liver Microsomes and S9 Corresponding author: Hao-Jie Zhu Ph.D. Department of Clinical Pharmacy University of Michigan College of Pharmacy 428 Church Street, Room 4565 Downloaded from Ann Arbor, MI 48109-1065 Tel: 734-763-8449, E-mail: [email protected] dmd.aspetjournals.org Number of words in Abstract: 250 at ASPET Journals on October 2, 2021 Number of words in Introduction: 776 Number of words in Discussion: 2304 2 DMD Fast Forward. Published on November 7, 2019 as DOI: 10.1124/dmd.119.089235 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 89235 Non-standard ABBreviations: DMEs, drug metabolism enzymes; HLM, human liver microsomes; HLS9, -

Gene Expression Atlas.Pptx
ArrayExpress Gene Expression Atlas Investigating gene expression patterns The Bioinformatics Roadshow – Rotterdam Erasmus Postgraduate school Molecular Medicine Ibrahim Emam Functional Genomics Group European Bioinformatics Institute ArrayExpress – two databases May 2, 2012 2 How we view experiments… • Given one experiment where the effect of a particular compound treatment is assayed from two different strains in four different tissue types. 02/05/2012 3 Examine profile of Saa4 in one experiment • With respect to Compound Treatment 02/05/2012 4 Examine profile of Saa4 in one experiment • With respect to Genotype 02/05/2012 5 Examine profile of Saa4 in one experiment • With respect to Organism Part (Tissue) 02/05/2012 6 Atlas construction Atlas construction - example Meta-analysis framework • For each experiment: • Identify differentially expressed genes between groups of samples • A gene is significantly differentially expressed if the combined F- statistic derived from all pairwise comparisons of the means of a gene's expression levels across factors has a sufficiently small adjusted p-value. • Score every condition/gene/experiment triplet – this score gives us the likelihood that this gene is differentially expressed for this condition in the given experiment • Correct these scores for multiple testing and make a cut-off – differentially expressed: yes/no • Repeat for all experiments 02/05/20129 Meta-analysis framework-cont’d • For every condition-gene pair count in how many experiments it is differentially expressed • The result is -

Functional Gene Clusters in Global Pathogenesis of Clear Cell Carcinoma of the Ovary Discovered by Integrated Analysis of Transcriptomes
International Journal of Environmental Research and Public Health Article Functional Gene Clusters in Global Pathogenesis of Clear Cell Carcinoma of the Ovary Discovered by Integrated Analysis of Transcriptomes Yueh-Han Hsu 1,2, Peng-Hui Wang 1,2,3,4,5 and Chia-Ming Chang 1,2,* 1 Department of Obstetrics and Gynecology, Taipei Veterans General Hospital, Taipei 112, Taiwan; [email protected] (Y.-H.H.); [email protected] (P.-H.W.) 2 School of Medicine, National Yang-Ming University, Taipei 112, Taiwan 3 Institute of Clinical Medicine, National Yang-Ming University, Taipei 112, Taiwan 4 Department of Medical Research, China Medical University Hospital, Taichung 440, Taiwan 5 Female Cancer Foundation, Taipei 104, Taiwan * Correspondence: [email protected]; Tel.: +886-2-2875-7826; Fax: +886-2-5570-2788 Received: 27 April 2020; Accepted: 31 May 2020; Published: 2 June 2020 Abstract: Clear cell carcinoma of the ovary (ovarian clear cell carcinoma (OCCC)) is one epithelial ovarian carcinoma that is known to have a poor prognosis and a tendency for being refractory to treatment due to unclear pathogenesis. Published investigations of OCCC have mainly focused only on individual genes and lack of systematic integrated research to analyze the pathogenesis of OCCC in a genome-wide perspective. Thus, we conducted an integrated analysis using transcriptome datasets from a public domain database to determine genes that may be implicated in the pathogenesis involved in OCCC carcinogenesis. We used the data obtained from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) DataSets. We found six interactive functional gene clusters in the pathogenesis network of OCCC, including ribosomal protein, eukaryotic translation initiation factors, lactate, prostaglandin, proteasome, and insulin-like growth factor. -

Comparative Transcriptomics Identifies Potential Stemness-Related Markers for Mesenchymal Stromal/Stem Cells
bioRxiv preprint doi: https://doi.org/10.1101/2021.05.25.445659; this version posted May 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Comparative Transcriptomics Identifies Potential Stemness-Related Markers for Mesenchymal Stromal/Stem Cells Authors Myret Ghabriel 1, Ahmed El Hosseiny 1, 2, Ahmed Moustafa*1, 2 and Asma Amleh*1, 2 Affiliations 1Biotechnology Program, American University in Cairo, New Cairo 11835, Egypt 2Department of Biology, American University in Cairo, New Cairo 11835, Egypt *Corresponding authors: Ahmed Moustafa [email protected] Asma Amleh [email protected]. Abstract Mesenchymal stromal/stem cells (MSCs) are multipotent cells residing in multiple tissues with the capacity for self-renewal and differentiation into various cell types. These properties make them promising candidates for regenerative therapies. MSC identification is critical in yielding pure populations for successful therapeutic applications; however, the criteria for MSC identification proposed by the International Society for Cellular Therapy (ISCT) is inconsistent across different tissue sources. In this study, we aimed to identify potential markers to be used together with the ISCT’s criteria to provide a more accurate means of MSC identification. Thus, we carried out a comparative analysis of the expression of human and mouse MSCs derived from multiple tissues to identify the common differentially expressed genes. We show that six members of the proteasome degradation system are similarly expressed across MSCs derived from bone marrow, adipose tissue, amnion, and umbilical cord. -

Genome-Wide Transcript and Protein Analysis Reveals Distinct Features of Aging in the Mouse Heart
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.28.272260; this version posted April 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Genome-wide transcript and protein analysis reveals distinct features of aging in the mouse heart Isabela Gerdes Gyuricza1, Joel M. Chick2, Gregory R. Keele1, Andrew G. Deighan1, Steven C. Munger1, Ron Korstanje1, Steven P. Gygi3, Gary A. Churchill1 1The Jackson Laboratory, Bar Harbor, Maine 04609 USA; 2Vividion Therapeutics, San Diego, California 92121, USA; 3Harvard Medical School, Boston, Massachusetts 02115, USA Corresponding author: [email protected] Key words for online indexing: Heart Aging Transcriptomics Proteomics eQTL pQTL Stoichiometry ABSTRACT Investigation of the molecular mechanisms of aging in the human heart is challenging due to confounding factors, such as diet and medications, as well limited access to tissues. The laboratory mouse provides an ideal model to study aging in healthy individuals in a controlled environment. However, previous mouse studies have examined only a narrow range of the genetic variation that shapes individual differences during aging. Here, we analyzed transcriptome and proteome data from hearts of genetically diverse mice at ages 6, 12 and 18 months to characterize molecular changes that occur in the aging heart. Transcripts and proteins reveal distinct biological processes that are altered through the course of natural aging. Transcriptome analysis reveals a scenario of cardiac hypertrophy, fibrosis, and reemergence of fetal gene expression patterns. -
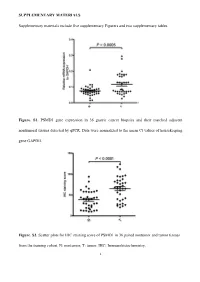
Supplementary Figures and Tables
SUPPLEMENTARY MATERIALS Supplementary materials include five supplementary Figurers and two supplementary tables. Figure. S1. PSMD1 gene expression in 36 gastric cancer biopsies and their matched adjacent nontumoral tissues detected by qPCR. Data were normalized to the mean Ct values of housekeeping gene GAPDH. Figure. S2. Scatter plots for IHC staining score of PSMD1 in 36 paired nontumor and tumor tissues from the training cohort. N: nontumor; T: tumor. IHC: Immunohistochemistry. 1 Figure. S3. Kaplan-Meier survival curve analysis of DFS for all 411 patients with gastric cancer according to the PSMD1 expression stratified by clinicopathological risk factors. P-values were calculated by the log-rank test. 2 Figure. S4. Kaplan-Meier survival curve analysis of OS for all 411 patients with gastric cancer according to the PSMD1 expression stratified by clinicopathological risk factors. P-values were calculated by the log-rank test. 3 Figure. S5. Extended model for prediction of DFS and OS in patients with GC based on PSMD1 expression. ROC analyses of sensitivity and specificity for prediction of a, b DFS and c, d OS by the combined model (TNM stage and PSMD1 expression combination), TNM stage model, andPSMD1 expression model. 4 Figure. S6. X-tile plots of the DFS and OS nomograms. Color intensity of the plot represents low (dark, black) to high (bright, red, or green) strength of association at each division. Red represents the inverse association between the PSMD1 expression and survival (DFS or OS), whereas green represents direct association. a DFS nomogram, b OS nomogram. 5 Table S1. Clinical characteristics of the 36 patients with gastric cancer. -

HBA2 Antibody Cat
HBA2 Antibody Cat. No.: 23-575 HBA2 Antibody Immunohistochemistry of paraffin-embedded mouse Immunohistochemistry of paraffin-embedded mouse heart using HBA2 antibody (23-575) at dilution of 1:100 brain using HBA2 antibody (23-575) at dilution of 1:100 (40x lens). (40x lens). Specifications HOST SPECIES: Rabbit SPECIES REACTIVITY: Mouse, Rat Recombinant fusion protein containing a sequence corresponding to amino acids 1-142 of IMMUNOGEN: human HBA2 (NP_000508.1). TESTED APPLICATIONS: IHC, WB WB: ,1:500 - 1:2000 APPLICATIONS: IHC: ,1:50 - 1:100 POSITIVE CONTROL: 1) Mouse liver September 30, 2021 1 https://www.prosci-inc.com/hba2-antibody-23-575.html PREDICTED MOLECULAR Observed: 13kDa WEIGHT: Properties PURIFICATION: Affinity purification CLONALITY: Polyclonal ISOTYPE: IgG CONJUGATE: Unconjugated PHYSICAL STATE: Liquid BUFFER: PBS with 0.02% sodium azide, 50% glycerol, pH7.3. STORAGE CONDITIONS: Store at -20˚C. Avoid freeze / thaw cycles. Additional Info OFFICIAL SYMBOL: HBA2 ALTERNATE NAMES: Hemoglobin subunit alpha, Alpha-globinHBH GENE ID: 3040 USER NOTE: Optimal dilutions for each application to be determined by the researcher. Background and References The human alpha globin gene cluster located on chromosome 16 spans about 30 kb and includes seven loci: 5'- zeta - pseudozeta - mu - pseudoalpha-1 - alpha-2 - alpha-1 - theta - 3'. The alpha-2 (HBA2) and alpha-1 (HBA1) coding sequences are identical. These genes differ slightly over the 5' untranslated regions and the introns, but they differ significantly over the 3' untranslated regions. Two alpha chains plus two beta chains constitute HbA, BACKGROUND: which in normal adult life comprises about 97% of the total hemoglobin; alpha chains combine with delta chains to constitute HbA-2, which with HbF (fetal hemoglobin) makes up the remaining 3% of adult hemoglobin. -

Transposable Elements As Sensors of SARS-Cov-2 Infection
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.25.432821; this version posted February 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Transposable elements as sensors of SARS-CoV-2 infection Matan Sorek1,2,*, Eran Meshorer1,2,* and Sharon Schlesinger3,* 1Department of Genetics, The Institute of Life Sciences, The Hebrew University of Jerusalem, Edmond J. Safra Campus, Jerusalem 9190100, Israel, 2Edmond and Lily Safra Center for Brain Sciences (ELSC), The Hebrew University of Jerusalem, Edmond J. Safra Campus, Jerusalem 9190100, Israel 3Department of Animal Sciences, Faculty of Agriculture, The Hebrew University of Jerusalem, Rehovot 7610001, Israel *Corresponding authors ([email protected]; [email protected]; [email protected]) 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.25.432821; this version posted February 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Transposable elements (TEs) are induced in response to viral infections. TEs induction triggers a robust and durable interferon (IFN) response, providing a host defense mechanism. Still, the connection between SARS-CoV-2 IFN response and TEs remained unexplored. Here, we analyzed TE expression changes in response to SARS-CoV-2 infection in different human cellular models. -

Additional File 3.Pdf
************ globins ************ >Pdu_Egb_A1a MNGITVFLILAMASASLADDCTQLDMIKVKHQWAEVYGVESNRQEFGLAVFKRFFVIHPD RSLFVNVHGDNVYSPEFQAHVARVLAGVDILISSMDQEAIFKAAQKHYADFHKSKFGEVPLVEFGTAMRDVLP KYVGLRNYDNDSWSRCYAYITSKVE >Pdu_Egb_A1b MKGLLVFLVLASVSASLASECSSLDKIKVKNQWA RIHGSPSNRKAFGTAVFKRFFEDHPDRSLFANVNGNDIYSADFQAHVQRVFGGLDILIVSLDQDDLFTAAKSH YSEFHKKLGDVPFAEFGVAFLDTLSDFLPLRDYNQDPWSRCYNYIIS >Pdu_Egb_A1c MNTVTVVLVLLG CIASAMTGDCNTLQRTKVKYQWSIVYGATDNRQAFGTLVWRDFFGLYPDRSLFSGVRGENIYSPEFRAHVVRV FAGFDILISLLDQEDILNSALAHYAAFHKQFPSIPFKEFGVVLLEALAKTIPEQFDQDAWSQCYAVIVAGVTA >Pdu_Egb_A1d_alpha MYQILSVAVLVLSCLALGTLGEEVCGPLERIKVQHQWVSVYGADHDRLKVSTL VWKDFFEHHPEERARFERVNSDNIFSGDFRAHMVRVFAGFDLLIGVLNEEEIFKSAMIHYTKMHNDLGVTTEI IKEFGKSIARVLPEFMDGKPDITAWRPCFNLIAAGVSE >Pdu_Egb_A1d_beta MYFSYFTAAASYLSVAVLVLSCLVQGILGEEVCGPLEKIKVQHQWASAYRGDHD RLKMSTLVWKDFFAHNPEERARFERVHSDDIYSGDFRAHMVRVFAGFDLLIGALNQEDIFRSAMIHYTKMHKK LGVTYEIGIEFGKSIGRVLPEFIDGKLDITAWRPCYKLIATGVDE >Pdu_Egb_A1d_gamma MYLSVAVLVLSCLALGTQGEEVCGPLEKIKVQHQWASAYRGDHDRLKMSTLVW KDFFAHHPEERARFERVHSDDIYSGDFRAHMVRVFAGFDLLIGVLNQDEIFKSAMIHYTKMHNDLGVKTEIVL EFGKSIARVLPDFIDGKPDITAWRPCFKLIAAGVSE >Pdu_Egb_A2 MNNLVILVGLLCLGLTSATKCGPL QRLKVKQQWAKAYGVGHERLELGIALWKSIFAQDPESRSIFTRVHGDDVRHPAFEAHIARVFNGFDRIISSLT DEDVLQAQLAHLKAQHIKLGISAHHFKLMRTGLSYVLPAQLGRCFDKEAWGSCWDEVIYPGIKSL >Pdu_Egb_B1 MLVLAVFVAALGLAAADQCCSIEDRNEVQALWQSIWSAENTGKRTIIGHQIFEELFDINP GTKDLFKRVNVEDTSSPEFEAHVLRVMNGLDTLIGVLDDPATGYSLITHLAEQHKAREGFKPSYFKDIGVALK RVLPQVASCFNPEAWDHCFNGFVEAITNKMNAL >Pdu_Egb_B2 MLVLVLSLAFLGSALAEDCCSAADRKTVLRDWQSVWSAEFTGRRVAIGTAIFEELFAIDA GAKDVFKNVAVDKPESAEWAAHVIRVINGLDLAINLLEDPRALKEELLHLAKQHRERDGVKAVYFDEIGRALL -

Anti-Hemoglobin Antibody (FITC) Product Number: AC15-0147-12
Anti-Hemoglobin Antibody (FITC) Product Number: AC15-0147-12 Overview Host Species: Goat Clonality: Polyclonal Purity: Hemoglobin Antibody (FITC) is affinity purified. The affinity purified antibody is then conjugated to the fluorescent dye FITC (fluorescein isothiocyanate). Conjugate: FITC Immunogen Type: Anti-hemoglobin antibody (FITC) was rasied against human hemoglobin. Physical Characteristics Amount: 0.05 mg Concentration: 1 mg/ml Volume: 0.05 ml Buffer: 10 mM Sodium Phosphate, 0.15 M NaCl, pH 7.2 with 0.05% (w/v) sodium azide. Storage: FITC conjugated antibody can be stored at 4?C for up to 18 months. For longer storage the conjugate can be stored at -20?C after adding 50% glycerol. Fluorescein conjugated antibodies should always be stored in the dark. Specificity Species Reactivity: Human Specificity: Human hemoglobin Target Information Gene ID Number(s): 3047 (human), 3039 (human), 3040 (human) Alternative Names: 3-prime alpha-globin gene; Alpha globin; alpha one globin; alpha-1 globin; Alpha-globin; Beta globin; CD113t C; CD31antibody (FITC); Erythremia, beta-globin type, included; Gamma 1 globin; Hb FAgamma; HBA 1; HBA 2; HBA; HBA_HUMAN; HBA1antibody (FITC); HBA2; HBB; Hbb-y; HBD; Hbe1; HBG 1antibody (FITC); HBG; HBG1; HBGA; HBGR; HBHantibody (FITC); Hemoglobin alpha 1; hemoglobin alpha 1 globin chain; Hemoglobinalpha chain; Hemoglobin alpha locus; Hemoglobin alpha locus 1antibody (FITC); hemoglobin alpha-1 chain; Hemoglobin beta; Hemoglobin beta chainantibody (FITC); Hemoglobin beta chain complex; Hemoglobin beta locus; Hemoglobingamma