Poll Res-16.Pmd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Maharashtra Tourism Development Corporation Ltd., Mumbai 400 021
WEL-COME TO THE INFORMATION OF MAHARASHTRA TOURISM DEVELOPMENT CORPORATION LIMITED, MUMBAI 400 021 UNDER CENTRAL GOVERNMENT’S RIGHT TO INFORMATION ACT 2005 Right to information Act 2005-Section 4 (a) & (b) Name of the Public Authority : Maharashtra Tourism Development Corporation (MTDC) INDEX Section 4 (a) : MTDC maintains an independent website (www.maharashtratourism. gov.in) which already exhibits its important features, activities & Tourism Incentive Scheme 2000. A separate link is proposed to be given for the various information required under the Act. Section 4 (b) : The information proposed to be published under the Act i) The particulars of organization, functions & objectives. (Annexure I) (A & B) ii) The powers & duties of its officers. (Annexure II) iii) The procedure followed in the decision making process, channels of supervision & Accountability (Annexure III) iv) Norms set for discharge of functions (N-A) v) Service Regulations. (Annexure IV) vi) Documents held – Tourism Incentive Scheme 2000. (Available on MTDC website) & Bed & Breakfast Scheme, Annual Report for 1997-98. (Annexure V-A to C) vii) While formulating the State Tourism Policy, the Association of Hotels, Restaurants, Tour Operators, etc. and its members are consulted. Note enclosed. (Annexure VI) viii) A note on constituting the Board of Directors of MTDC enclosed ( Annexure VII). ix) Directory of officers enclosed. (Annexure VIII) x) Monthly Remuneration of its employees (Annexure IX) xi) Budget allocation to MTDC, with plans & proposed expenditure. (Annexure X) xii) No programmes for subsidy exists in MTDC. xiii) List of Recipients of concessions under TIS 2000. (Annexure X-A) and Bed & Breakfast Scheme. (Annexure XI-B) xiv) Details of information available. -

District Census Handbook, Thane
CENSUS OF INDIA 1981 DISTRICT CENSUS HANDBOOK THANE Compiled by THE MAHARASHTRA CENSUS DIRECTORATE BOMBAY PRINTED IN INDIA BY THE MANAGER, GOVERNMENT CENTRAL PRESS, BOMBAY AND PUBLISHED BY THE DIRECTOR, GOVERNMENT PRINTING, STATIONERY AND PUBLICATIONS, MAHARASHTRA STATE, BOMBAY 400 004 1986 [Price-Rs.30·00] MAHARASHTRA DISTRICT THANE o ADRA ANO NAGAR HAVELI o s y ARABIAN SEA II A G , Boundary, Stote I U.T. ...... ,. , Dtstnct _,_ o 5 TClhsa H'odqllarters: DCtrict, Tahsil National Highway ... NH 4 Stat. Highway 5H' Important M.talled Road .. Railway tine with statIOn, Broad Gauge River and Stream •.. Water features Village having 5000 and above population with name IIOTE M - PAFU OF' MDKHADA TAHSIL g~~~ Err. illJ~~r~a;~ Size', •••••• c- CHOLE Post and Telegro&m othce. PTO G.P-OAJAUANDHAN- PATHARLI [leg .... College O-OOMBIVLI Rest House RH MSH-M4JOR srAJE: HIJHWAIY Mud. Rock ." ~;] DiStRICT HEADQUARTERS IS ALSO .. TfIE TAHSIL HEADQUARTERS. Bo.ed upon SUI"'Ye)' 0' India map with the Per .....ion 0( the Surv.y.,.. G.,.roI of ancIo © Gover..... ,,, of Incfa Copyrtgh\ $8S. The territorial wat.,. rilndia extend irato the'.,a to a distance 01 tw.1w noutieol .... III80sured from the appropf'iG1. ba .. tin .. MOTIF Temples, mosques, churches, gurudwaras are not only the places of worship but are the faith centres to obtain peace of the mind. This beautiful temple of eleventh century is dedicated to Lord Shiva and is located at Ambernath town, 28 km away from district headquarter town of Thane and 60 km from Bombay by rail. The temple is in the many-cornered Chalukyan or Hemadpanti style, with cut-corner-domes and close fitting mortarless stones, carved throughout with half life-size human figures and with bands of tracery and belts of miniature elephants and musicians. -

Study and Development of Manekpur Village
International Journal of Recent Development in Engineering and Technology Website: www.ijrdet.com (ISSN 2347-6435(Online) Volume 10, Issue 3, March 2021) Study and Development of Manekpur Village Prince Mediwala1, Urvika Munoor2, Amit Chauhan3 1,2,3Department of Civil Engineering, Laxmi Institute of Technology, Sarigam, Gujarat, India [email protected],[email protected],[email protected] Abstract—Rural areas need drastic changes in areas like As a part of designs for part I, we have given designs of infrastructure, credit availability, literacy, poverty eradication, following structures like, Rainwater Harvesting Plant as a etc. The schemes that are already in place with the aim of rural sustainable design, Public Toilet as a physical design, Post- development need a new outlook and proper updating. Accordingly, the government needs to act for the upliftment of Office as a social design, Community Hall as a socio-cultural rural India. The main objective of this paper is to give a design idea design, Bank with ATM facility as smart design and Gram about the methodology and the development needed in the Panchayat as a heritage design. allocated village. For this particular project we have taken ―Manekpur‖ village, which is located in Valsad district of Gujarat II. PROJECT METHODOLOGY state. We have done techno-economical survey and gap analysis for the village. From the survey we got to know about the conditions of Introduction to VISHWAKARMA YOJANA existing government structure, sanitation structure, economical Literature Reviews structure and so based on that survey we have given the design of various structure for the prosperity of the village and to make it To Do Techno-Economical Survey one step more closure towards a smart village. -

Organizational Set up of CBEC
Organizational Set Up of CBEC Updated as on 19.02.2015 [Training Material for departmental use] E-BOOK On Organizational Set Up of CBEC After Cadre Restructuring Prepared by NACEN, RTI, Kanpur Page 0 Organizational Set Up of CBEC Note: In this E-book, attempts have been made to know about Organizational Set up of CBEC after Cadre Restructuring. It is expected that it will help the new entrants into the service. Though all efforts have been made to make this document error free, but it is possible that some errors might have crept into the document. If you notice any errors or if you have any suggestion to improve this document, the same may be brought to the notice to the NACEN, RTI, Kanpur on the Email addresses: [email protected] or [email protected] (Email address of ADG, RTI, NACEN, Kanpur). This may not be a prefect E-book and all are requested to assist us to make it better. Prepared by NACEN, RTI, Kanpur Page 1 Organizational Set Up of CBEC INDEX Organization & Functions .................................................................................................................. 3 Composition and Functions of Central Board of Excise and Customs .............................................. 3 Attached Offices of CBEC................................................................................................................. 3 Structure of Field Formations under CBEC....................................................................................... 4 Territorial Jurisdiction of Central Excise Commissionerates -

7 and Construction of Panagar Bypass Including New Connectivit
Brief Project Summary Project: Development of Vadodara Mumbai Expressway (Phase-II) from km.26.32 to km.104.700 (km.390.864 of NH-8) of Main Expressway in the state of Maharashtra Brief Project Summary 1. The proposed alignment of Mumbai – Vadodara Expressway (Phase-II), a greenfield alignment, starts from proposed chainage km 26+320 (near Vasai in the state of Maharashtra) and ends at chainage km 104+700 (km 390+864 of NH-8 near Talasari in the state of Maharashtra). 2. Total length of the Phase-II alignment is 78.38 km and proposed Right of Way (RoW) is 100 / 120 m 3. The proposed alignment is passing through 4 talukas (Vasai, Palghar, Dahanu and Talasari) of Palghar district in the state of Maharashtra. The alignment is passing through plain and rolling terrain with cultivated and barren areas 4. The project road crosses 51 villages in Palghar district. The predominant land use along the alignment is agricultural followed by vegetation cover including forest. 5. The proposed expressway passes through Dahanu Taluka Eco Fragile Zone for a length of 27.8 km from km 68+400 to km 96+200. An area of 346.44 ha is to be acquired in Dahanu Taluka. 6. The proposed expressway falls within 10 km of the Tungareshwar Wildlife Sanctuary and the nearest distance is 1.8 km. 7. CRZ is present along the alignment for a total length of 3.575 km in Vasai (from Km 29.900 to km 30.434 & km 30.462 to km 30.800) and Palghar Taluka (km 30.800 to km 32.100; km 35.430 to km 36.833). -

Pincode Officename Mumbai G.P.O. Bazargate S.O M.P.T. S.O Stock
pincode officename districtname statename 400001 Mumbai G.P.O. Mumbai MAHARASHTRA 400001 Bazargate S.O Mumbai MAHARASHTRA 400001 M.P.T. S.O Mumbai MAHARASHTRA 400001 Stock Exchange S.O Mumbai MAHARASHTRA 400001 Tajmahal S.O Mumbai MAHARASHTRA 400001 Town Hall S.O (Mumbai) Mumbai MAHARASHTRA 400002 Kalbadevi H.O Mumbai MAHARASHTRA 400002 S. C. Court S.O Mumbai MAHARASHTRA 400002 Thakurdwar S.O Mumbai MAHARASHTRA 400003 B.P.Lane S.O Mumbai MAHARASHTRA 400003 Mandvi S.O (Mumbai) Mumbai MAHARASHTRA 400003 Masjid S.O Mumbai MAHARASHTRA 400003 Null Bazar S.O Mumbai MAHARASHTRA 400004 Ambewadi S.O (Mumbai) Mumbai MAHARASHTRA 400004 Charni Road S.O Mumbai MAHARASHTRA 400004 Chaupati S.O Mumbai MAHARASHTRA 400004 Girgaon S.O Mumbai MAHARASHTRA 400004 Madhavbaug S.O Mumbai MAHARASHTRA 400004 Opera House S.O Mumbai MAHARASHTRA 400005 Colaba Bazar S.O Mumbai MAHARASHTRA 400005 Asvini S.O Mumbai MAHARASHTRA 400005 Colaba S.O Mumbai MAHARASHTRA 400005 Holiday Camp S.O Mumbai MAHARASHTRA 400005 V.W.T.C. S.O Mumbai MAHARASHTRA 400006 Malabar Hill S.O Mumbai MAHARASHTRA 400007 Bharat Nagar S.O (Mumbai) Mumbai MAHARASHTRA 400007 S V Marg S.O Mumbai MAHARASHTRA 400007 Grant Road S.O Mumbai MAHARASHTRA 400007 N.S.Patkar Marg S.O Mumbai MAHARASHTRA 400007 Tardeo S.O Mumbai MAHARASHTRA 400008 Mumbai Central H.O Mumbai MAHARASHTRA 400008 J.J.Hospital S.O Mumbai MAHARASHTRA 400008 Kamathipura S.O Mumbai MAHARASHTRA 400008 Falkland Road S.O Mumbai MAHARASHTRA 400008 M A Marg S.O Mumbai MAHARASHTRA 400009 Noor Baug S.O Mumbai MAHARASHTRA 400009 Chinchbunder S.O -

The Kisan Sabha and Adivasi Struggles in Thane District After 1947 Saqib Khan*
RESEARCH ARTICLE The Kisan Sabha and Adivasi Struggles in Thane District after 1947 Saqib Khan* Abstract: This article traces the history of the All India Kisan Sabha (AIKS) in Thane district, Maharashtra, after 1947, that is, after the Warli Adivasi revolt of 1947. It is based on oral narratives by AIKS activists and leaders and on secondary sources. After the Warli revolt, the AIKS in Thane district mobilised the people around issues of land, forests, water, wages, and other livelihood issues. The article documents and analyses the nature of this mobilisation, the demands raised by the AIKS, and the extent to which these demands achieved success. Our evidence suggests mobilisation was successful because the people posed continuous challenges to multiple forms of exploitation by means of mobilisation by the AIKS on issues of Adivasi livelihood, and as a result of the initiation of welfare programmes through participation in local-level electoral politics. Keywords: Adivasi struggles, Thane district, All India Kisan Sabha, oral history, agrarian relations in Maharashtra, Scheduled Tribes, land rights, Forest Rights Act. Introduction Historically, during the period of British colonial rule as well as after India gained Independence, the Adivasi people of India have been victims of economic and social exploitation and marginalisation. A recent contribution to the literature has characterised the principal means of exploitation of the Adivasis as being alienation from land and the denial of access to common property resources by violent, coercive displacement, and a corresponding loss of livelihoods (Karat and Rawal 2014, Shah 2004). British colonialism effected and aggravated the alienation of the Adivasi people from land and forests in a number of ways (Desai 1979, Prasad 2006–7, Chattopadhyay 2012). -
PALGHAR (Version
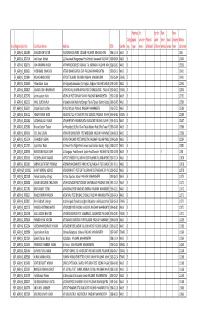
Handica Ex- Earthq Part- Non- Categ pped service Project uake time Sport creamy Marks Sl no Registration No Candidate Name Address DOB Gender ory type men affected affecte emplo sman layer obtained 1 P_AGRI_KS_0060984 VISHAL KISHOR SUTAR TALASARI HADALPADA TALASARI PALGHAR MAHARASHTRA 1988-11-14 MALE ST 140.1 2 P_AGRI_KS_0057106 Sunil Vasant Mahale 223 Kasatwadi Bhangrepadai Post Shiroshi Kasatwadi PALGHAR MAHARASHTRA1989-08-26 MALE ST 136.04 3 P_AGRI_KS_0039715 RAM ATMARAM WAGH AT PIMPALGAON POST VASHALA TAL MOKHADA PALGHAR MAHARASHTRA1988-05-01 MALE ST 135.03 4 P_AGRI_KS_0009651 JAYESH BABU DHANGADA AT-ZARI DHANGADPADA ZARI PALGHAR MAHARASHTRA 1990-03-11 MALE ST 134.01 5 P_AGRI_KS_0029844 ARUNA DHANJI DHODI AT POST TALASARI TALASARI PALGHAR MAHARASHTRA 1993-05-04 FEMALE ST 134.01 6 P_AGRI_KS_0018349 Pritam Baban Govari At- Kolgaon(vankaspada) Tq- Palghar, Kolgaon PALGHAR MAHARASHTRA1991-03-05 MALE ST 129.95 7 P_AGRI_KS_0008807 RANJANA TANHI BHARBHARE AT CHINCHALE BHARBHARPADA POST DHUNDALWADI PALGHAR 1992-06-12 MAHARASHTRAFEMALE ST 128.93 8 P_AGRI_KS_0015763 Sachin Jayaram Nathe AT CHALNI POST SAYVAN SAYVAN PALGHAR MAHARASHTRA 1992-01-08 MALE ST 127.92 9 P_AGRI_KS_0062152 RAHUL SURESH VALVI At Sambha Valvi Pada Post Dongari Taluka Talasari District Palghar 1986-03-02 Sambha PALGHARMALE MAHARASHTRAST 125.89 10 P_AGRI_KS_0030011 Vinayak Vasant Jadhav At Post Pathardi Pathardi PALGHAR MAHARASHTRA 1992-07-21 MALE ST 125.89 11 P_AGRI_KS_0056722 HEMLATA ARUN BHOIR HOUSE NO.722, AT DHUKTAN POST GOWADE PALGHAR MAHARASHTRA1995-06-03 FEMALE ST -

Full Operational Alert
y k y cm DREAM PROFESSION SMOG RAISES FEARS CHINA FACES ALIENATION Actor Yami Gautam says her job gives her Delhi residents are enduring one of the worst Xi Jinping appears to be worried over the ample scope to travel and explore places spells of air pollution in years, raising risks adverse fallout of his belligerent posed by the coronavirus foreign policy as much as she would love LEISURE | P2 TWO STATES | P7 INTERNATIONAL P10 VOLUME 10, ISSUE 219 | www.orissapost.com BHUBANESWAR | TUESDAY, NOVEMBER 10 | 2020 12 PAGES | `4.00 IRREGULAR by MANJUL Laxmipur BDO seeks money on DM’s China, Russia refuse behalf; audio viral POST NEWS NETWORK Jeypore, Nov 9: In a bizarre de- velopment, Laxmipur block de- velopment officer (BDO) in Koraput to endorse Biden win district allegedly demanded money from local sarpanches and exec- AGENCIES President utive officers under Jeypore block Donald Biden gets down to limits Sunday. Beijing, Nov 9: China and Russia Trump According an audio clip that So, who’s going Monday declined to congratulate Joe makes his work; Trump digs in has gone viral, the officer, Dillip Tirtol and Balasore to fool us for the Biden as the winner of the US presi- stand Patnaik, demanded `30,000 from next five years? WASHINGTON: US President-elect Joe dential election, saying the outcome of clear, Wang Biden took the first steps towards mov- sarpanches and executive officers, the American polls should be deter- said: “We ing into the White House in 73 days, as saying the amount would be given bypoll results today mined by the country's laws and pro- will follow Donald Trump again refused to admit to District Collector. -

Sr No Customer Name Customer Address Zone Region
Sr No Customer Name Customer Address Zone Region Location District State Class of Mkt Urban Rural Highway 1 MS/HSD- MILAN AUTO SERVICE MUMBAI AGRA ROAD BHIWANDI THANA 421302 West VASHI Retail RO BHIWANDI MH-THANE Maharastra Retail B Urban 2 MS/HSD A D ADAJANIA & CO 25 RAJARAMMOHAN ROY ROAD MUMBAI 400004 West MUMBAI Retail RO MUMBAI MH-MUMBAI Maharastra Retail A Urban 3 MS/HSD- AUTOMOBILE CENTRE OPP KHIRA NAGAR S V ROAD SANTA- CRUZ MUMBAI 400054 West MUMBAI Retail RO MUMBAI MH-MUMBAI Maharastra Retail A Urban 4 MS/HSD AUTOMOBILE RESORT, DEONAR NEAR TELECOM FACTORY SION-TROMBAY ROAD DEONAR 400074 West MUMBAI Retail RO NA MH-MUMBAI Maharastra Retail A Urban 5 MS/HSD BAXIS SINGH & CO., KURLA HPC DEALER AGRA ROAD KURLA MUMBAI 400070 West MUMBAI Retail RO MUMBAI MH-MUMBAI Maharastra Retail A Urban 6 MS/HSD BRIGHT AUTOMOBILES H P C DEALERS SAFED POOL MOHILI ANDHERI -KURLA ROAD MUMBAI 400072 West MUMBAI Retail RO MUMBAI MH-MUMBAI Maharastra Retail A Urban 7 MS/HSD CAR CARE CENTRE-GOREGAON WESTERN EXPRESS HIGHWAY AAREY FLYOVER PAHADI 400063 West MUMBAI Retail RO MUMBAI MH-MUMBAI Maharastra Retail A Urban 8 MS/HSD CITY SERVICE CADELL ROAD, PRABHADEVI MUMBAI 28 400028 West MUMBAI Retail RO PRABHADEVI MH-MUMBAI Maharastra Retail A Urban 9 MS/HSD COTTON GREEN SERVICE STATION HPC DEALERS 2,JACKERIA BUNDER RD, COTTON GREEN 400033 West MUMBAI Retail RO COTTON GREEN MH-MUMBAI Maharastra Retail A Urban 10 MS/HSD DIAMOND AUTO SERVICE, CHEMBUR HPC DEALERS NEAR AKBARALLY'S CHEMBUR 400071 West MUMBAI Retail RO CHEMBUR MH-MUMBAI Maharastra Retail A Urban 11 MS/HSD DINESH AUTO SERVICE, GHATKOPAR B/6 LBS MARG GHATKOPAR WEST 400086 West MUMBAI Retail RO GHATKOPAR WEST MH-MUMBAI Maharastra Retail A Urban 12 MS/HSD DOABA AUTOMOBILES HPC DEALER 365, SANE GURUJI MAARG, AGRIPADA 400011 West MUMBAI Retail RO AGRIPADA MH-MUMBAI Maharastra Retail A Urban 13 MS/HSD NAT. -

State Disaster Management Plan
Disaster Management Plan Maharashtra State Disaster Management Plan State Disaster Management Authority Mantralaya, Mumbai April, 2016 Disaster Management Unit Relief and Rehabilitation Department Government of Maharashtra Contents PART – I Chapter – 1 1. Introduction Page No 1.1 Background ............................................................................................... 1 1.2 Vision ....................................................................................................... 1 1.3 Objective of the Plan ................................................................................. 2 1.4 Themes ..................................................................................................... 2 1.5 Approach ................................................................................................... 2 1.6 Strategy ..................................................................................................... 3 1.7 Scope of the Plan ...................................................................................... 3 1.8 Authority and Reference ........................................................................... 4 1.9 Level of Disasters ..................................................................................... 4 1.10 Plan Development and Activation ............................................................. 4 1.11 Review/update of DM Plan ....................................................................... 5 1.12 Plan Testing ............................................................................................. -

Research Report
Research Report SDG monitoring tool for 3 Gram Panchayats in Uddyam – Talasari Integrated Skills Development Project By Pankaj Shrivastav CEO Tathyashodh Development Consultants Dehradun [email protected] Feb 2018 1 TABLE OF CONTENTS Executive Summary .................................................................................................................... 4 Abbreviations Used .................................................................................................................... 5 A. Evolution and importance of the Sustainable Development Goals ........................................ 6 1. The Millennium Development Goals and gaps in their achievement ............................................................6 2. The Sustainable Development Goals - SDGs .................................................................................................7 3. The importance of India in achieving global SDGs ........................................................................................7 4. Need for localisation of the SDGS ................................................................................................................8 5. Approach to the SDGs of the Government of India ......................................................................................8 B. Background to the study area and project ............................................................................. 9 1. The Uddyam project context .......................................................................................................................9