Clairfield Medtech Market Survey 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The United States District Court for the Southern. District of Florida
UNITEDBTATES DISTRICT COURT SOUTHERN DISTRICT OF FLORIDA ADMINISTRATIVE ORDER 2021-46 INRE: ·FILED BY CW D.C. EXEMPTION OF FEES TO ACCESS PACER May 20, 2021 FOR EMILY M. HOMER. ANGE\.AI:. NOBl..E Cl.ERKU.S. CIST..C'i'. S. 0. OF Fl.A - MIA The United States District Court for the Southern.District of Florida maintains case information online with the Public Access to Court Electronic Records (PACER) website. The Court desires to encourage the public's use of the database and to· continue to promote public access and to avoid unreasonable burdens on both users and court staffin retrievmg data. The Court finds that Emily M. Homer, as an individual researcher associated with an educationalinstitution, falls within the class of users listed in thefee schedule as being eligible for a fee exemption. Additionally, Ms. Homer has demonstrated that an exemption is necessary in order to avoid unreasonable burdens and to promote public access to information. It is hereby ORDERED AND ADJUDGED that Emily M. Homer shall be exempt from the . payment of fees for access via PACER to the electronic case files maintained in this Court, to the extent such use is incurred in the course of her research, specifically an examination of corporate deterrence and recidivism in 174 public organizations listed on Appendix 1 that signed deferred prosecution and non-prosecution agreements with federal prosecutors between 1992-2020. Ms. Homer will use this information to determine the frequency of violations to gauge the effectiveness of corporate agreements in preventing recidivism. Ms. Homer shall not be exempt from the payment of fees incurred in connection with other · uses of the PACER system in this Court. -

Cboe Germany 30 Index BDE30P
Cboe Germany 30 Index BDE30P Page 1 August 2021 Cboe Exchange The Cboe DE 30 index aims to be comprised of the largest 30 German issuers. This is a price return index. Objective The index is designed for use in the creation of index tracking funds, derivatives and as a performance benchmark. Investability Liquidity Transparency Availability Stocks are selected and Stocks are screened to Uses a transparent, rules-based Calculation is based on weighted to ensure that the ensure that the index is construction process. Index price and total return index is investable. tradable. Rules are freely available on the methodologies, both real cboe.com/europe/indices -time, intra-second and website. end of day. Statistics Index ISIN Ticker RIC Currency Cboe Germany 30 DE000SLA2P95 BDE30P .BDE30P EUR Cboe Germany 30 - net DE000SLA2QH0 BDE30N .BDE30N EUR Volatility Volatility (1y) 0.1741 Returns(%) 1M 3M 6M YTD 1Y 3Y 5Y BDE30P 1.81 2.58 11.45 11.58 17.02 13.3 24.82 BDE30N 1.81 2.65 13.3 13.68 19.32 21.0 38.83 Top 5 Performers Country 1 month return % MERCK KGAA GERMANY 17.08 INFINEON TECHNOLOGIES AG GERMANY 11.63 RWE AG GERMANY 10.07 HANNOVER RUCK SE GERMANY 9.68 MUNICH REINSURANCE COMPANY GERMANY 8.34 Historical Performance Chart 250% 200% 150% 100% 50% 0% 2011 2013 2014 2016 2017 2018 2020 2021 Cboe Germany 30 (EUR) Cboe Germany Mid Cap 50 (EUR) Cboe Germany Small Cap 50 (EUR) Cboe.com | ©Cboe | /CboeGlobalMarkets | /company/cboe © 2021 Cboe Exchange, Inc. All Rights Reserved. -
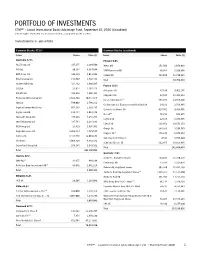
Portfolio of Investments
PORTFOLIO OF INVESTMENTS CTIVP® – Lazard International Equity Advantage Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.6% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 6.9% Finland 1.0% AGL Energy Ltd. 437,255 4,269,500 Metso OYJ 153,708 2,078,669 ASX Ltd. 80,181 4,687,834 UPM-Kymmene OYJ 36,364 1,106,808 BHP Group Ltd. 349,229 9,021,842 Valmet OYJ 469,080 11,570,861 Breville Group Ltd. 153,867 2,792,438 Total 14,756,338 Charter Hall Group 424,482 3,808,865 France 9.5% CSL Ltd. 21,611 4,464,114 Air Liquide SA 47,014 7,452,175 Data#3 Ltd. 392,648 1,866,463 Capgemini SE 88,945 11,411,232 Fortescue Metals Group Ltd. 2,622,808 30,812,817 Cie de Saint-Gobain(a) 595,105 24,927,266 IGO Ltd. 596,008 1,796,212 Cie Generale des Etablissements Michelin CSA 24,191 2,596,845 Ingenia Communities Group 665,283 2,191,435 Electricite de France SA 417,761 4,413,001 Kogan.com Ltd. 138,444 2,021,176 Elis SA(a) 76,713 968,415 Netwealth Group Ltd. 477,201 5,254,788 Legrand SA 22,398 1,783,985 Omni Bridgeway Ltd. 435,744 1,234,193 L’Oreal SA 119,452 38,873,153 REA Group Ltd. 23,810 1,895,961 Orange SA 298,281 3,106,763 Regis Resources Ltd. -

ASN Kidney Week 2020 Reimagined: Disclosures Page 1
10/14/2020 ASN Kidney Week 2020 Reimagined: Disclosures Page 1 Last Name First Name Nothing Employer Consultancy Ownership Interest Research Funding Honoraria Patents or Inventions Scientific Advisor or Membership Speakers Bureau Other Interests or Relationships to Disclose Abdel-Kader Khaled Vanderbilt University Medical Center BMC Nephrology; CJASN NKF Education committee; NIDDK Health IT work group Abudayyeh Ala University of Texas MD Anderson Cancer Center Adler Sharon Retrophin; Bristol Myers Squibb; Bayer; Retrophin; Bayer; ChemoCentryx; Omeros; Zyversa Bayer; Zyversa; Retrophin; AstraZeneca; Morphosys Retrophin; Bayer Pharmaceuticals; Zyversa; KDIGO; KRN; NephCure Kidney International AstraZeneca; ChemoCentryx; Omeros; Zyversa; Therapeutics; Calliditas; Morphosys AstraZeneca; Morphosys Foundation; Karger Publishers Morphosys; Karger Afkarian Maryam University of California, Davis Afrouzian Marjan University of Texas Medical Branch Alexion Pharmaceuticals; Banff Foundation Afshinnia Farsad Agarwal Anupam University of Alabama at Birmingham Dynamed - review content related to AKI for Goldilocks Therapeutics Genzyme/Sanofi Fabry Fellowship Award Univ Southern California, Vanderbilt, Emory, Akebia Editorial Board of AJP Renal, Kidney Int and My wife, Lisa Curtis, will be President-elect Dynamed and review updated materials prepared by Lab Investigation; invited to serve on for Women in Nephrology (2018-2019). Dynamed editorial team for AKI topics. Akebia - Advisory board of Goldilocks Therapeutics, Expert Panel to review new therapeutics -

SECURITIES and EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K REPORT of FOREIGN PRIVATE ISSUER PURSUANT to RULE 13A-16 OR
SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13A-16 OR 15D-16 OF THE SECURITIES EXCHANGE ACT OF 1934 For the month of July 2021 Commission file number: 001-32749 FRESENIUS MEDICAL CARE AG & Co. KGaA (Translation of registrant's name into English) Else-Kröner Strasse 1 61346 Bad Homburg Germany (Address of principal executive offices) Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F. Form 20-F Form 40-F Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ____ Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ____ FRESENIUS MEDICAL CARE AG & Co. KGaA Interim Report of Financial Condition and Results of Operations for the three and six months ended June 30, 2021 and 2020 Page FINANCIAL INFORMATION Management’s discussion and analysis Forward-looking statements ..................................................................................................................................... 1 Financial condition and results of operations ........................................................................................................ 3 Overview ..................................................................................................................................................................... 3 Discussion of measures .......................................................................................................................................... -

Press Release: Fresenius Medical Care
Media Contact Michael Gavin T +49 6172 609-2978 [email protected] Press Release Media Contact for North America Brad Puffer T +1 781-699-3331 [email protected] Contact for analysts and investors Dr. Dominik Heger T +49 6172 609-2601 [email protected] September 24, 2020 The Power of Ideas and the people behind them: Fresenius Medical Care publishes 2020 Global Annual Medical Report Fresenius Medical Care, the world’s leading provider of products and services for people with chronic kidney failure, today announced publication of its 2020 Global Annual Medical Report. Titled “The Power of Ideas: Ingenuity, Optimism, and the Future of Global Healthcare,” it advances the worldwide conversation about the continuing evolution of healthcare and underlines the role Fresenius Medical Care can play in transforming care for chronically ill patients. “This year’s report focuses on the ideas, ingenuity and optimism that are transforming healthcare,” said Franklin W. Maddux, MD, Global Chief Medical Officer of Fresenius Medical Care. “We look at ways to enhance patient choice, new frontiers in connected health, the promise of artificial intelligence, and other important topics including our global response to COVID-19. Our 2020 report also shows the remarkable diversity of people behind the ideas that are helping us evolve our healthcare systems to recognize the unique lifetime journey of each kidney disease patient.” The 2020 Global Annual Medical Report comprises 24 chapters by nearly 40 authors from across the company, representing some of Fresenius Medical Care’s best thought leadership on a wide range of topics. -

From Good Intentions to Real Results – Corporate Citizenship of Germany's DAX 30 Companies
FROM GOOD INTENTIONS TO REAL RESULTS Corporate Citizenship of Germany’s DAX 30 Companies + Relevance: Why is Corporate Citizenship a CEO topic? + Trends: What are current and future expectations regarding Corporate Citizenship? + Current practice: How do DAX 30 companies engage in societal causes? + Good practices: What does good Corporate Citizenship look like? + Advice: How can you revise your own Corporate Citizenship approach? 03 01 EXECUTIVE CONTENTS SUMMARY 01 EXECUTIVE SUMMARY __ PAGE 03 We consider Corporate Citizenship as the contributions of businesses to society through the combination of core business activities, CORPORATE CITIZENSHIP social investment and philanthropy, and __ 02 IN A WORLD OF SOCIETAL CHALLENGES PAGE 06 participation in the public policy process. (World Economic Forum, 2002) 03 METHODOLOGY OF THE STUDY __ PAGE 10 04 CORPORATE CITIZENSHIP OF DAX 30 COMPANIES __ PAGE 13 The world is facing dramatic challenges ranging from social people from both the for-profit and non-profit sectors still INTEGRATED CORPORATE CITIZENSHIP: inequality and climate change to healthcare systems that argue that companies should focus on their business and 05 SCHNEIDER ELECTRIC are stretched to the limit. Recognizing these challenges, limit their social activities to paying taxes and simply making the United Nations with their Sustainable Development donations to Non-Governmental Organizations (NGOs). __ AS GOOD PRACTICE EXAMPLE PAGE 20 Goals (SDGs) are calling upon all members of society to play their part. However, this view is slowly changing in different parts of the world, with new regulations being put in place to encourage With their expertise and resources, companies can play a key and enforce a more active role of companies in addressing __ SUMMARY AND RECOMMENDATIONS PAGE 21 role in addressing these societal challenges: the fact that the societal changes. -

180706 AR DAX 30 Event Presentation Engl FINAL
DAX 30 supervisory board study 2018 Selected results July 2018 Jens-Thomas Pietralla & Dr. Thomas Tomkos Board & CEO Practice Private and Confidential The following assessment and evaluation is based on publicly available biographical data *) on supervisory board members of DAX 30 and MDAX companies. Obviously, the effectiveness of supervisory boards also depends on the board culture, the personalities of their members and their constructive dialogue. *) Data sources: bioGraphies made available by companies, invitations to annual meetings, annual reports, voting results from annual meetings; additional externally available bioGraphical data where necessary Private and Confidential 2 Preview... § As expected, the ”super election year 2018” has resulted in siGnificant chanGes: 89 seats were up for election, of which 42 were filled by new candidates § EiGht new diGital directors add critical expertise to the DAX 30 boards. As of 2018 more than half of the companies can rely on this experience § For the first time the share of women amonG shareholder representatives in DAX 30 exceeds 30% § Equivalent representation of women in chairperson and committee positions is still laGGinG behind § While the number of foreiGn board members in DAX 30 declined sliGhtly, the companies are able to attract experienced female board members, especially from the anglophone world § The cross-linkage of “Germany Inc.” is shifting: there are less supervisory board members with more than one DAX 30 mandate; however, several current DAX 30 executive board members take on DAX 30 supervisory board sets for the first time § The average grade of all DAX 30 supervisory boards according to the German school grading system improves aGain: from 2.3 to 2.2 § Lufthansa, Daimler, Bayer and MunichRe lead the rankinG in 2018. -

1 Bekanntmachung in Bezug Auf Die Folgenden Long Bzw. Short
Bekanntmachung in Bezug auf die folgenden Long bzw. Short Mini Future Turbo Optionsscheine der Goldman, Sachs & Co. Wertpapier GmbH Hinsichtlich der unten genannten Long bzw. Short Mini Future Turbo Optionsscheine ist gemäß der Optionsscheinbedingungen ein Knock-Out Ereignis eingetreten, da die jeweilige Knock-Out Barriere erreicht oder unterschritten (bei Long Optionsscheinen) bzw. erreicht oder überschritten (bei Short Optionsscheinen) wurde. Die betroffenen Optionsscheine werden wie folgt abgerechnet: Datum des Knock-Out WKN Basiswert Optionstyp Knock-Out Barriere Knock-Out Auszahlungsbetrag je Ereignisses Optionsschein in EUR GH5C65 DAX® (Performance Index) Short EUR 15.304,73 29. April 2021 1,55 GH5CEH FTSE 100 Index Short GBP 7.000 29. April 2021 1,6 GH5CDP FTSE 100 Index Short GBP 7.010 29. April 2021 1,64 GH092Q CAC 40® Index Short EUR 6.329,329 29. April 2021 1,28 GH5CDE FTSE 100 Index Short GBP 6.980 29. April 2021 1,59 GH091Q CAC 40® Index Short EUR 6.344,004 29. April 2021 1,28 GH5CDF FTSE 100 Index Short GBP 6.990 29. April 2021 1,63 GH092S CAC 40® Index Short EUR 6.322,166 29. April 2021 1,28 GH01C8 AEX-Index® Short EUR 716,911 29. April 2021 0,15 GH5CDH FTSE 100 Index Short GBP 7.000 29. April 2021 1,68 GH5CDN FTSE 100 Index Short GBP 6.980 29. April 2021 1,67 GF9WRD EURO STOXX® Banks (Price EUR) Index Short EUR 91,007 29. April 2021 0,02 GH581W Nordex SE Long EUR 25,13 29. April 2021 1,19 GH5C8S ENI S.p.A. -

Glance 2021 Forward Thinking Healthcare
At a Glance 2021 Forward Thinking Healthcare Fresenius invested €748 million in research and development last year. Sales and Earnings in € millions 2020 2019 Growth Fresenius is a global healthcare group offering high-quality products Sales 36,277 35,409 2 % and services for dialysis, hospitals, and outpatient treatment. With over 300,000 employees in more than 100 countries around the EBIT 4,612 4,688 -2 % globe and annual sales exceeding €36 billion, Fresenius is one of the EBIT margin 12.7 % 13.2 % world’s leading healthcare companies. Net income 1,796 1,879 -4 % At Fresenius, the patient always comes fi rst. For more than 100 Operating cash fl ow 6,549 4,263 54 % years now we have been working to save lives and improve the quality of life of our patients. A clear focus on innovation and effi ciency Employees 2020 2019 has helped us to make high-quality healthcare accessible to a Employees (Dec. 31) 311,269 294,134 6 % steadily increasing number of people. Yet we never get complacent about our successes, and never stop looking for better solutions. This is how Fresenius is contributing to medical progress and better patient care. “Forward Thinking Healthcare” captures our commitment to the future: better medicine for more people. 03 Our year 2020 In battle against the coronavirus New biosimilar The COVID-19 pandemic confronted Fresenius Kabi applied for Dancing against COVID Fresenius with unprecedented regulatory approval of its Fresenius Helios and its challenges. The company took second biosimilar in the employees took up the extensive measures to ensure U.S. -

Expert Opinion on the Use of Cladribine Tablets in Clinical Practice
Expert Opinion on the Use of Cladribine METHODS • The consensus program was based on a multi-step modified Delphi • A comprehensive literature review was performed for each question. The Tablets in Clinical Practice methodology, which took place between April 2018 and April 2019. level of evidence was assessed and agreed by the SC.4 • A Steering Committee (SC) of nine international MS experts led the program. • A questionnaire was developed by the SC with draft answers based on The SC in turn was supported by an extended faculty (EF, n=33) of practicing available evidence from the literature review, combined with their expert P. Soelberg Sørensen1, D. Centonze2, G. Giovannoni3, X. Montalban4,5, D. Selchen5, P. Vermersch6, H. Wiendl7, B. Yamout8, neurologists caring for MS patients. A total of 19 countries were represented opinion, where evidence was lacking. The questionnaire was completed H. Salloukh9, P. Rieckmann10 in the program. The role of the EF was to review available evidence, remotely via an on-line platform by the EF. complete a questionnaire and finally vote on draft recommendations. 1Danish Multiple Sclerosis Center, Department of Neurology, University of Copenhagen and Rigshospitalet, Copenhagen, Denmark; 2Unit of GET POSTER PDF • The results from the questionnaire were incorporated into draft clinical Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy; 3Blizard Institute, Barts and The London School of Medicine and Dentistry, Copies of this poster obtained • The SC identified practical clinical questions concerning the use of cladribine recommendations, which were then voted on by the SC and EF members. 4 Queen Mary University of London, London, UK; Department of Neurology, Neuroimmunology, Multiple Sclerosis Centre of Catalonia (Cemcat), Vall through QR (Quick Response) tablets and prioritized the most important 11 questions to be addressed, d’Hebron University Hospital, Barcelona, Spain; 5University of Toronto, Division of Neurology, St. -

Download Social Media Radar Here!
Social Media Radar Social Media Radar. Life-Science-Industry. Healthcare. Namics. A Merkle Company 1 Social Media Radar Social Media. Life Science. Healthcare Marketing. Success. Digital Communication. Ranking. Twitter. Facebook. Instagram. YouTube. Likes. Shares. Tweets. Assets. KPI. Tools. Analysis. Namics. A Merkle Company 2 Social Media Radar Table of Contents 4 Methodology – About the Social Media Radar 8 Management summary – The life-science-industry in the social web 10 In detail: Facebook – Emotions on Facebook are inevitable 11 Ranking Facebook 12 Lessons learned 14 In detail: Twitter – The most relevant channel of the life-science-industry 15 Ranking Twitter 16 Lessons learned 18 In detail: Instagram – Need for action on the blogger-platform 19 Ranking Instagram 20 Lessons learned 22 In detail: YouTube – No video, no social-media-strategy 23 Ranking YouTube 24 Lessons learned 26 Conclusion – Social media offers a big potential for life-science-brands 28 Glossary 29 References 32 Contact 3 Social Media Radar Methodology About the Social Media Radar The Social Medie Radar by Namics analyzes the top-selling companies in the pharmaceutical-, chemistry- and medical technology industry with head offices located in Germany. The channels Facebook, Twitter, Instagram and YouTube are the focus of the analysis. Their popularity and the use for a broad customer communication in a natural scientific environment were crucial factors for the choice of these mediums. In addition, the visibility of the individual companies on the social web between May 1st, 2017 and April 30th, 2018 was a deciding criterion for the inclusion into the study. Primarily german company profiles were considered; if those were not available, the international profile was accessed.