Expert Opinion on the Use of Cladribine Tablets in Clinical Practice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Verily Forms Strategic Alliances with Novartis, Otsuka, Pfizer and Sanofi
Verily Forms Strategic Alliances with Novartis, Otsuka, Pfizer and Sanofi to Transform Clinical Research Leading biopharmaceutical organizations join Project Baseline initiative to engage more patients and clinicians in research and speed evidence generation South San Francisco, CA -- May 21, 2019 -- Verily, an Alphabet company, today announced strategic alliances with Novartis (NYSE: NVS), Otsuka (NYSE: OTSKY), Pfizer Inc. (NYSE: PFE) and Sanofi (EURONEXT: SAN, NASDAQ: SNY) to develop digitally innovative, patient-centered clinical research programs using Project Baseline’s evidence generation platform and tools. The Baseline Platform is designed to engage more patients and clinicians in research, increase the speed and ease of conducting studies and collect more comprehensive, higher quality data, including outside the four walls of a clinic. Across the United States, the number of people participating in clinical research -- including clinical trials and observational studies -- is less than 10% of the population.1 In addition to low participation, challenges in research include data fragmentation, inefficient operations and limited value for patients. Alongside academic research institutions, patient-advocacy groups and health systems, Verily and its industry partners aim to implement a more patient-centric, technology-enabled approach to research, and increase the number and diversity of clinical research participants. They will also explore novel approaches to generating real-world evidence using the Baseline Platform to collect, organize and activate health information from electronic health records, sensors and other digital sources. Over the coming years, Novartis, Otsuka, Pfizer and Sanofi each plan to launch clinical studies leveraging the platform across diverse therapeutic areas, such as cardiovascular disease, oncology, mental health, dermatology and diabetes. -

312.1.Full.Pdf
Ann Rheum Dis: first published as 10.1136/annrheumdis-2021-eular.1066 on 19 May 2021. Downloaded from 312 Scientific Abstracts Acknowledgements: This study was funded by Novartis Pharma AG. The found many DEGs from baseline with GUS treatment and none with PBO. These authors thank Richard Karpowicz, PhD, of Health Interactions, Inc, for providing included genes related to B-, T-, NK-, and plasma cells (increased by GUS) and medical writing support/editorial support, which was funded by Novartis Pharma- neutrophils, monocytes, eosinophils, and macrophages (decreased by GUS), ceuticals Corporation, East Hanover, NJ, in accordance with Good Publication suggestive of a partial normalization of immune cell composition in whole blood. Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Conclusion: Using whole transcriptome profiling, we detected DEGs in blood Disclosure of Interests: Gurjit S. Kaeley Consultant of: Novartis Pharmaceuti- samples obtained from PsA pts vs. healthy controls, suggesting a dysregulation cals Corporation, Georg Schett Speakers bureau: AbbVie, Bristol Myers Squibb, of immune cell profiles in PsA. The majority of these disease-associated genes Celgene, Janssen, Eli Lilly, Novartis, and Pfizer, Consultant of: AbbVie, Bristol were modulated by GUS, with directionality toward a normalization of whole Myers Squibb, Celgene, Janssen, Eli Lilly, Novartis, and UCB, Grant/research blood transcriptomic signatures. support from: Bristol Myers Squibb, Celgene, GSK, Eli Lilly, and Novartis, Philip REFERENCES: G Conaghan Consultant of: or Speakers bureau: AbbVie, AstraZeneca, Bristol [1] Deodhar A et al. Lancet. 2020;395:1115. Myers Squibb, Eli Lilly, EMD Serono, Flexion Therapeutics, Galapagos, Gilead, [2] Mease P et al. Lancet. 2020;395:1126. Novartis, and Pfizer, Grant/research support from: UK National Institute for Health Research (NIHR) Leeds Biomedical Research Centre, Dennis McGona- Table 1. -

CA Signed Novartis 315 Settlement Agreement Signed California
STATE SETTLEMENT AGREEMENT I. PARTIES This Settlement Agreement (the "Agreement") is entered into between the State of California ("the State") and Novartis Pharmaceuticals Corporation ("Novartis"), collectively, "the Parties." II. PREAMBLE As a preamble to this Agreement, the Parties agree to the following: A. At all relevant times, Novartis, (a subsidiary of Novartis International AG, which is headquartered in Basel, Switzerland) was a corporation with its principal place of business in East Hanover, New Jersey, distributed, marketed and/or sold pharmaceutical products in the United States, including drugs sold under the trade names of Lotrel, Valturna, Starlix, Tekamlo, Diovan, Diovan HCT, Tekturna, Tekturna HCT, Exforge, and Exforge HCT (the "Covered Drugs"). B. On January 5, 2011 , Oswald Bilotta, (the "Relater") filed a sealed qui tam action, which was subsequently amended on October 19, 2012, as of right on April 3, 2013 (pursuant to an unopposed motion dated March 21 , 2013), and on July 10, 2013 (pursuant to a stipulation dated July 8, 2013) (the "Civil Action") in the United States District Court for the Southern District of New York captioned United States of America et al. , ex rel. Oswald Bilotta et al. v. Novartis Pharmaceuticals Corporation Civil Action No. 1 l-cv-00071 , alleging, inter alia, that Novartis violated the False Claims Act ("FCA") and the Anti-Kickback Statute, 42 U.S.C. §I320a-7b(b) (the "AKS"), by paying doctors remuneration to prescribe the drugs Lotrel, Valturna, Starlix, Tekturna, Tekturna HCT, Diovan, Diovan HCT, Exforge, and Exforge HCT through the mechanism ofspeaker program honoraria and related misconduct. Novartis-315 Page 1 of 19 On April 26, 2013, the United States intervened in the Civil Action against Novartis by filing a Notice of Election to Intervene and Complaint-in-Intervention, in which it is asserted that claims against Novartis under the FCA and common law. -

AMGEN INC. V. SANOFI
Case: 20-1074 Document: 159 Page: 1 Filed: 06/21/2021 NOTE: This order is nonprecedential. United States Court of Appeals for the Federal Circuit ______________________ AMGEN INC., AMGEN MANUFACTURING, LIMITED, AMGEN USA, INC., Plaintiffs-Appellants v. SANOFI, AVENTISUB LLC, FKA AVENTIS PHARMACEUTICALS INC., REGENERON PHARMACEUTICALS INC., SANOFI-AVENTIS U.S. LLC, Defendants-Appellees ______________________ 2020-1074 ______________________ Appeal from the United States District Court for the District of Delaware in Nos. 1:14-cv-01317-RGA, 1:14-cv- 01349-RGA, 1:14-cv-01393-RGA, 1:14-cv-01414-RGA, Judge Richard G. Andrews. ______________________ JEFFREY A. LAMKEN, MoloLamken LLP, Washington, DC, filed a petition for rehearing en banc for plaintiffs-ap- pellants. Also represented by SARAH JUSTINE NEWMAN, MICHAEL GREGORY PATTILLO, JR.; SARA MARGOLIS, New York, NY; EMILY JOHNSON, ERICA S. OLSON, STEVEN TANG, STUART WATT, WENDY A. WHITEFORD, Amgen Inc., Thou- sand Oaks, CA; KEITH HUMMEL, Cravath Swaine & Moore LLP, New York, NY; WILLIAM G. GAEDE, III, McDermott Case: 20-1074 Document: 159 Page: 2 Filed: 06/21/2021 2 AMGEN INC. v. SANOFI Will & Emery LLP, Menlo Park, CA; CHRISTOPHER B. MEAD, Schertler Onorato Mead & Sears LLP, Washington, DC; JAMES L. HIGGINS, MELANIE K. SHARP, Young, Cona- way, Stargatt & Taylor, LLP, Wilmington, DE. Plaintiff- appellant Amgen Inc. also represented by SARAH CHAPIN COLUMBIA, McDermott, Will & Emery LLP, Boston, MA; LAUREN MARTIN, Quinn Emanuel Urquhart & Sullivan LLP, Boston, MA. MATTHEW WOLF, Arnold & Porter Kaye Scholer LLP, Washington, DC, filed a response for defendants-appellees. Also represented by VICTORIA REINES; DAVID K. BARR, DANIEL REISNER, New York, NY; DEBORAH E. -
Event Calendar
Published on Novartis (https://www.novartis.com) Home > Printer-friendly PDF > Event Calendar Event Calendar View recent presentations and learn more about upcoming events. Disclaimer: Disclaimer: The information in the presentations on these pages was factually accurate on the date of publication. These presentations remain on the Novartis website for historical purposes only. Novartis assumes no responsibility to update the information to reflect subsequent developments. Readers should not rely upon the information in these pages as current or accurate after their publication dates. Tab: Upcoming Events Third quarter results 2021 Oct 26, 2021 Basel, Switzerland Recent Events [1] ESG Investor Day Sep 30, 2021 Watch the webcast [2] Download the podcast (MP3 66 MB) [3] Access our interactive slide deck (PDF 9.6 MB) [4] Read the media release [5] Novartis ESG Investor Day 2021 6 key takeaways [6] Bernstein Strategic Decisions Conference Sep 23, 2021 Baader Investment Conference Sep 21, 2021 Bank of America Merrill Lynch Global Healthcare Virtual Conference Sep 15, 2021 Morgan Stanley Global Healthcare Conference Sep 13, 2021 Citi's GEMS Virtual Conference 2021 Sep 8, 2021 Second quarter results 2021 Jul 21, 2021 Basel, Switzerland Media release English (PDF 0.4 MB) [7] Deutsch (PDF 0.4 MB) [8] Français (PDF 0.4 MB) [9] Interim financial report (PDF 0.5 MB) [10] Watch the webcast [11] Read the presentation transcript [12] Download the podcast (MP3 45 MB) [13] Download the interactive presentation (PDF 4.5 MB) [14] Novartis ESG Update - July -

The United States District Court for the Southern. District of Florida
UNITEDBTATES DISTRICT COURT SOUTHERN DISTRICT OF FLORIDA ADMINISTRATIVE ORDER 2021-46 INRE: ·FILED BY CW D.C. EXEMPTION OF FEES TO ACCESS PACER May 20, 2021 FOR EMILY M. HOMER. ANGE\.AI:. NOBl..E Cl.ERKU.S. CIST..C'i'. S. 0. OF Fl.A - MIA The United States District Court for the Southern.District of Florida maintains case information online with the Public Access to Court Electronic Records (PACER) website. The Court desires to encourage the public's use of the database and to· continue to promote public access and to avoid unreasonable burdens on both users and court staffin retrievmg data. The Court finds that Emily M. Homer, as an individual researcher associated with an educationalinstitution, falls within the class of users listed in thefee schedule as being eligible for a fee exemption. Additionally, Ms. Homer has demonstrated that an exemption is necessary in order to avoid unreasonable burdens and to promote public access to information. It is hereby ORDERED AND ADJUDGED that Emily M. Homer shall be exempt from the . payment of fees for access via PACER to the electronic case files maintained in this Court, to the extent such use is incurred in the course of her research, specifically an examination of corporate deterrence and recidivism in 174 public organizations listed on Appendix 1 that signed deferred prosecution and non-prosecution agreements with federal prosecutors between 1992-2020. Ms. Homer will use this information to determine the frequency of violations to gauge the effectiveness of corporate agreements in preventing recidivism. Ms. Homer shall not be exempt from the payment of fees incurred in connection with other · uses of the PACER system in this Court. -

Cboe Germany 30 Index BDE30P
Cboe Germany 30 Index BDE30P Page 1 August 2021 Cboe Exchange The Cboe DE 30 index aims to be comprised of the largest 30 German issuers. This is a price return index. Objective The index is designed for use in the creation of index tracking funds, derivatives and as a performance benchmark. Investability Liquidity Transparency Availability Stocks are selected and Stocks are screened to Uses a transparent, rules-based Calculation is based on weighted to ensure that the ensure that the index is construction process. Index price and total return index is investable. tradable. Rules are freely available on the methodologies, both real cboe.com/europe/indices -time, intra-second and website. end of day. Statistics Index ISIN Ticker RIC Currency Cboe Germany 30 DE000SLA2P95 BDE30P .BDE30P EUR Cboe Germany 30 - net DE000SLA2QH0 BDE30N .BDE30N EUR Volatility Volatility (1y) 0.1741 Returns(%) 1M 3M 6M YTD 1Y 3Y 5Y BDE30P 1.81 2.58 11.45 11.58 17.02 13.3 24.82 BDE30N 1.81 2.65 13.3 13.68 19.32 21.0 38.83 Top 5 Performers Country 1 month return % MERCK KGAA GERMANY 17.08 INFINEON TECHNOLOGIES AG GERMANY 11.63 RWE AG GERMANY 10.07 HANNOVER RUCK SE GERMANY 9.68 MUNICH REINSURANCE COMPANY GERMANY 8.34 Historical Performance Chart 250% 200% 150% 100% 50% 0% 2011 2013 2014 2016 2017 2018 2020 2021 Cboe Germany 30 (EUR) Cboe Germany Mid Cap 50 (EUR) Cboe Germany Small Cap 50 (EUR) Cboe.com | ©Cboe | /CboeGlobalMarkets | /company/cboe © 2021 Cboe Exchange, Inc. All Rights Reserved. -

Investor Presentation
Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Q1 2021 Results Investor presentation 1 Investor Relations │ Q1 2021 Results Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” or similar expressions, or by express or implied discussions regarding potential new products, potential new indications for existing products, potential product launches, or regarding potential future revenues from any such products; or regarding the impact of the COVID-19 pandemic on certain therapeutic areas including dermatology, ophthalmology, our breast cancer portfolio, some newly launched brands and the Sandoz retail and anti-infectives business, and on drug development operations; or regarding potential future, pending or announced transactions; regarding potential future sales or earnings of the Group or any of its divisions; or by discussions of strategy, plans, expectations or intentions; or regarding the Group’s liquidity or cash flow positions and its ability to meet its ongoing financial obligations and operational needs; or regarding our collaboration with Molecular Partners to develop, manufacture and commercialize potential medicines for the prevention and treatment of COVID- 19 and our joining of the industry-wide efforts to meet global demand for COVID-19 vaccines and therapeutics by leveraging our manufacturing capacity and capabilities to support the production of the Pfizer-BioNTech vaccine and to manufacture the mRNA and bulk drug product for the vaccine candidate CVnCoV from CureVac. -

R&D Update Call 2020
R&D Update Call 2020 Luciano Rossetti, Global Head of Research & Development Rehan Verjee, Head of the Global Innovative Medicine Franchises & President of EMD Serono Joern-Peter Halle, Head of Research Klaus Edvardsen, Head of Oncology Development September 25, 2020 Disclaimer Publication of Merck KGaA, Darmstadt, Germany. In the United States and Canada the group of companies affiliated with Merck KGaA, Darmstadt, Germany operates under individual business names (EMD Serono, Millipore Sigma, EMD Performance Materials). To reflect such fact and to avoid any misconceptions of the reader of the publication certain logos, terms and business descriptions of the publication have been substituted or additional descriptions have been added. This version of the publication, therefore, slightly deviates from the otherwise identical version of the publication provided outside the United States and Canada. 2 Disclaimer Cautionary Note Regarding Forward-Looking Statements and financial indicators This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements. -
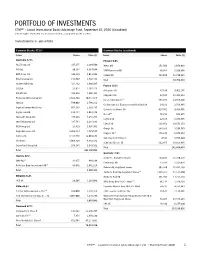
Portfolio of Investments
PORTFOLIO OF INVESTMENTS CTIVP® – Lazard International Equity Advantage Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.6% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 6.9% Finland 1.0% AGL Energy Ltd. 437,255 4,269,500 Metso OYJ 153,708 2,078,669 ASX Ltd. 80,181 4,687,834 UPM-Kymmene OYJ 36,364 1,106,808 BHP Group Ltd. 349,229 9,021,842 Valmet OYJ 469,080 11,570,861 Breville Group Ltd. 153,867 2,792,438 Total 14,756,338 Charter Hall Group 424,482 3,808,865 France 9.5% CSL Ltd. 21,611 4,464,114 Air Liquide SA 47,014 7,452,175 Data#3 Ltd. 392,648 1,866,463 Capgemini SE 88,945 11,411,232 Fortescue Metals Group Ltd. 2,622,808 30,812,817 Cie de Saint-Gobain(a) 595,105 24,927,266 IGO Ltd. 596,008 1,796,212 Cie Generale des Etablissements Michelin CSA 24,191 2,596,845 Ingenia Communities Group 665,283 2,191,435 Electricite de France SA 417,761 4,413,001 Kogan.com Ltd. 138,444 2,021,176 Elis SA(a) 76,713 968,415 Netwealth Group Ltd. 477,201 5,254,788 Legrand SA 22,398 1,783,985 Omni Bridgeway Ltd. 435,744 1,234,193 L’Oreal SA 119,452 38,873,153 REA Group Ltd. 23,810 1,895,961 Orange SA 298,281 3,106,763 Regis Resources Ltd. -

BIOGEN INTERNATIONAL GMBH, Plaintiff-Appellant
Case: 20-1373 Document: 41 Page: 1 Filed: 04/21/2020 United States Court of Appeals for the Federal Circuit ______________________ BIOGEN INTERNATIONAL GMBH, Plaintiff-Appellant v. BANNER LIFE SCIENCES LLC, Defendant-Appellee ______________________ 2020-1373 ______________________ Appeal from the United States District Court for the District of Delaware in No. 1:18-cv-02054-LPS, Chief Judge Leonard P. Stark. ______________________ Decided: April 21, 2020 ______________________ JAMES B. MONROE, Finnegan, Henderson, Farabow, Garrett & Dunner, LLP, Washington, DC, for plaintiff-ap- pellant. Also represented by PAUL WILLIAM BROWNING, J. MICHAEL JAKES, LAURA POLLARD MASUROVSKY, JASON LEE ROMRELL. KYLE MUSGROVE, Parker Poe Adams & Bernstein LLP, Charlotte, NC, for defendant-appellee. Also represented by JOHN WORTHINGTON BATEMAN, ELIZABETH CROMPTON, SCOTT A. CUNNING, II, Washington, DC. ______________________ Case: 20-1373 Document: 41 Page: 2 Filed: 04/21/2020 2 BIOGEN INTERNATIONAL GMBH v. BANNER LIFE SCIENCES LLC Before LOURIE, MOORE, and CHEN, Circuit Judges. LOURIE, Circuit Judge. Biogen International GmbH (“Biogen”) appeals from a judgment of the United States District Court for the Dis- trict of Delaware that Banner Life Sciences LLC (“Banner”) does not infringe the extended portion of U.S. Patent 7,619,001 (the “’001 patent”), extended under the patent term restoration provisions of the Hatch-Waxman Act, Pub. L. No. 98-417, § 201, 98 Stat. 1585, 1598 (as codified at 35 U.S.C. § 156 (2018)). Biogen Int’l GmbH v. Banner Life Scis. LLC, No. 18-2054-LPS, 2020 WL 109499 (D. Del. Jan. 7, 2020) (“Decision”). Because the scope of a patent term extension under 35 U.S.C. -

Human Sequencing Resource with UK Biobank
January 8, 2018 Regeneron Forms Consortium of Leading Life Sciences Companies to Accelerate Largest Widely-Available 'Big Data' Human Sequencing Resource with UK Biobank By end of 2019, Regeneron plans to sequence the exomes of all 500,000 people within the UK Biobank resource, all with associated health records, creating an unprecedented resource linking human genetic variations to human biology and disease AbbVie, Alnylam Pharmaceuticals, AstraZeneca, Biogen and Pfizer will join Regeneron to co-fund one of the industry's most ambitious 'pre-competitive' research efforts Exome sequencing data and findings will be openly available to other researchers TARRYTOWN, N.Y., Jan. 8, 2018 /PRNewswire/ -- Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN), along with new collaborators AbbVie, Alnylam Pharmaceuticals, AstraZeneca, Biogen and Pfizer Inc., today announced the formation of a major 'pre-competitive' consortium to fund the generation of genetic exome sequence data from the 500,000 volunteer participants who make up the UK Biobank health resource. The newly announced collaborators will each commit $10 million to enable a dramatic acceleration of sequencing timelines, and additional companies are considering joining the consortium. Regeneron will conduct the sequencing effort. The sequencing data will be paired with detailed, de-identified medical and health records within the UK Biobank resource, including enhanced measures such as brain, heart and body imaging, to create an unparalleled resource for linking human genetic variations to human biology and disease. It was originally planned that sequencing of all 500,000 samples in the UK Biobank would be completed by 2022, with the first 50,000 people sequenced during 2017 with funding from Regeneron and GlaxoSmithKline.