Analysis of Grass Carp Dynamics to Optimize Hydrilla Control in an Appalachian Reservoir by Matthew A. Weberg Thesis Submitted T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fries Hydroelectric Project FERC Project No
ENVIRONMENTAL ASSESSMENT FOR HYDROPOWER LICENSE Fries Hydroelectric Project FERC Project No. 2883-009 Virginia Federal Energy Regulatory Commission Office of Energy Projects Division of Hydropower Licensing 888 First Street, NE Washington, D.C. 20426 December 2020 TABLE OF CONTENTS TABLE OF CONTENTS ..................................................................................................... i LIST OF FIGURES ............................................................................................................ iii LIST OF TABLES .............................................................................................................. iv ACRONYMS AND ABBREVIATIONS ............................................................................ v 1.0 INTRODUCTION .................................................................................................... 1 1.1 APPLICATION ............................................................................................. 1 1.2 PURPOSE OF ACTION AND NEED FOR POWER .................................. 1 1.2.1 Purpose of Action .............................................................................. 1 1.2.2 Need for Power .................................................................................. 3 1.3 STATUTORY AND REGULATORY REQUIREMENTS ......................... 4 1.4 PUBLIC REVIEW AND COMMENT ......................................................... 4 1.4.1 Scoping .............................................................................................. 4 -

Cla Ytor Lake Festival Claytor Lake State
In the Fall of 2014, FOCL was invited to partner with the local chapter of The U.S. Coast Guard Auxiliary to teach a Boater/Water Safety Class at the Hensel Eckman YMCA in Pulaski. Allison Hunter, Director of the “Y”, Sharon Eifred of the Coast Guard Auxiliary, and Cheri Strenz from FOCL, developed a plan to introduce Boater and Water Safety to young children. Volunteer instructors from FOCL included, Laura Walters, Larry Bandolin, and Cheri Strenz. For six weeks, second grade students from the elementary schools in Pulaski County attend swim classes at the “Y”. One of these sessions is to teaching what a family should know before going out on a boat for the day. This includes the equipment required for boating, general rules of navigation, and how to identify and select a Coast Guard Approved life jacket. Many of these children have made numerous trips to Claytor Lake and are aware that a life jacket should be worn. Sadly, many did not know that a life jacket is required to be worn by all children 12 and under. They also learned, by trying on various sizes, that a properly fitted Coast Guard Approved lifejacket is essential to providing the proper amount of life saving protection. This critical information about boating and being around the water is important having a fun and safe day at Claytor Lake, or any other body of water. FOCL is looking forward to continued participation in this worthwhile program. This year the Pulaski County Chamber of Commerce awarded its first Non-Profit Award for outstanding work in the community at The Draper Claytor Lake Community Programs Community Lake Claytor Mercantile. -

Dunkard's Bottom: Memories on the Virginia Landscape, 1745 to 1940
DUNKARD’S BOTTOM: MEMORIES ON THE VIRGINIA LANDSCAPE, 1745 TO 1940 HISTORICAL INVESTIGATIONS FOR SITE 44PU164 AT THE CLAYTOR HYDROELECTRIC PROJECT PULASKI COUNTY, VIRGINIA FERC PROJECT NO. 739 Prepared for: Prepared by: Appalachian Power Company S&ME, Inc. 40 Franklin Road 134 Suber Road Roanoke, Virginia 24011 Columbia, South Carolina 29210 and and Kleinschmidt Associates, Inc. Harvey Research and Consulting 2 East Main Street 4948 Limehill Drive Strasburg, Pennsylvania 17579 Syracuse, New York 13215 Authors: Heather C. Jones, M.A., and Bruce Harvey, Ph.D. Final Report – July 2012 History of Dunkard’s Bottom Appalachian Power Company Claytor Hydroelectric Project July 2012 TABLE OF CONTENTS TABLE OF CONTENTS ................................................................................................................ 2 TABLE OF FIGURES .................................................................................................................... 2 INTRODUCTION .......................................................................................................................... 3 DUNKARD‘S BOTTOM ............................................................................................................... 3 The Dunkards ......................................................................................................................................... 4 William Christian ................................................................................................................................. 12 The Cloyd Family -

Pulaski County Serve Pulaski County Unserved Last Mile Broadband - Phase One
Application to DHCD Submitted through CAMS Pulaski County Serve Pulaski County Unserved Last Mile Broadband - Phase One Application ID: 64508312019091002 Application Status: Pending Program Name: Virginia Telecommunications Initiative 2020 Organization Name: Pulaski County Organization Address: 143 Third St, NW Pulaski, VA 24301-4900 Profile Manager Name: Jared Linkous Profile Manager Phone: (540) 980-7710 Profile Manager Email: [email protected] Project Name: Serve Pulaski County Unserved Last Mile Broadband - Phase One Project Contact Name: Jonathan Sweet Project Contact Phone: (540) 980-7710 Project Contact Email: [email protected] Project Location: 143 Third Street Pulaski, VA 24301-4900 Project Service Area: Pulaski County Total Requested Amount: $612,475.00 Required Annual Audit Status: Pending Review 9/4/2019 1:50:08 PM Pages: 1 of 17 Application to DHCD Submitted through CAMS Pulaski County Serve Pulaski County Unserved Last Mile Broadband - Phase One Budget Information: Cost/Activity Category DHCD Request Other Funding Total Telecommunications $612,475.00 $410,000.00 $1,022,475.00 Construction $342,900.00 $235,600.00 $578,500.00 Construction Related Soft Costs $122,049.00 $80,400.00 $202,449.00 Other: Equipment, Redundancy $147,526.00 $94,000.00 $241,526.00 Total: $612,475.00 $410,000.00 $1,022,475.00 Budget Narrative: The total cost of the last mile broadband shovel ready project is $1,022,475. The project includes network design, engineering, and configuration for a cost of $ 100,000; Network Operations Center -

Table of Contents
Appalachian Power Company Claytor Hydroelectric Project FERC No. 739 Debris Management Plan June 2009 TABLE OF CONTENTS Description Page SUMMARY…………………………………………………………………… 1 1.0 Introduction……………………………………………………………….. 2 1.1 Project Lands and Waters…………………………………………. 2 1.2 Debris Study Objectives and Conclusion………………………… 2 2.0 Debris Management………………………………………………………. 3 2.1 Existing Efforts…………………………………………………… 3 2.2 Beneficial Debris…………………………………………………. 4 3.0 Management Measures…………………………………………………… 4 3.1 Debris Removal………………………………………………….. 4 3.2 Offload/Disposal Sites……………………………………………. 5 3.3 Volunteer Lake Clean-up Efforts………………………………… 5 3.4 Education…………………………………………………………. 5 3.5 Coordination……………………………………………………… 6 3.6 Costs……………………………………………………………… 6 3.7 Schedule………………………………………………………….. 6 4.0 Modifications to Plan…………………………………………………….. 7 5.0 Report ……………………………………………………………………. 7 SUMMARY The Claytor Project (No. 739) is licensed to Appalachian Power Company (Appalachian) and is a conventional hydroelectric project located on the New River in Pulaski County, Virginia. The project boundary for the Claytor Project generally follows the 1850 foot contour around the perimeter of the reservoir. Elevations are referred to National Geodetic Vertical Datum (NGVD). The purpose of this Debris Management Plan is to identify debris removal and control measures within the project boundary for the Claytor Project that maintains the aesthetic values, reduce access difficulties, and reduce boating hazards associated with floating debris while also benefiting -
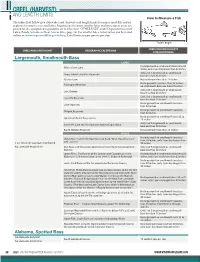
Creel & Length Limit Table
CREEL (HARVEST) AND LENGTH LIMITS How to Measure a Fish The tables that follow give statewide creel (harvest) and length limits for major sport fish and -ex ceptions for major rivers and lakes. Regulations for many smaller lakes and boat access areas are posted on site, and posted regulations are in effect (see “OTHER USES” under Department-owned Lakes, Ponds, Streams or Boat Access Sites, page 14). For smaller lakes, information can be found online at: www.virginiawildlife.gov/fishing. Creel limits are per person per day. Total Length CREEL OR LENGTH LIMITS CREEL AND LENGTH LIMIT GEOGRAPHIC EXCEPTIONS FOR EXCEPTIONS Largemouth, Smallmouth Bass LAKES No largemouth or smallmouth bass 16 to 24 Briery Creek Lake inches, only 1 per day longer than 24 inches Only 2 of 5 largemouth or smallmouth Buggs Island Lake/Kerr Reservoir bass less than 14 inches Claytor Lake No smallmouth less than 14 inches No largemouth bass less than 12 inches; Flannagan Reservoir no smallmouth bass less than 15 inches Only 2 of 5 largemouth or smallmouth Lake Gaston bass less than 14 inches Only 2 of 5 largemouth or smallmouth Leesville Reservoir bass less than 14 inches No largemouth or smallmouth bass less Lake Moomaw than 12 inches No largemouth or smallmouth bass less Philpott Reservoir than 12 inches No largemouth or smallmouth bass 12 to Quantico Marine Base waters 15 inches Only 2 of 5 largemouth or smallmouth Smith Mt. Lake and its tributaries below Niagara Dam bass less than 14 inches South Holston Reservoir No smallmouth less than 15 inches RIVERS No largemouth or smallmouth bass less Clinch River–within the boundaries of Scott, Wise, Russell or Taze- than 20 inches, only 1 per day longer than well counties 5 per day in the aggregate (combined) 20 inches No statewide length limits Dan River and tributaries downstream from the Union Street Dam, Only 2 of 5 largemouth or smallmouth Danville bass less than 14 inches James River–Confluence of the Jackson and Cowpasture rivers No largemouth or smallmouth bass 14 to 22 (Botetourt Cty) downstream to the 14th St. -

2020 Claytor Lake Fisheries Report from the Department of Wildlife
Claytor Lake: More than a Wide Spot in the New River John R. Copeland, Fisheries Biologist, Blacksburg Office April 1, 2020 Imagine yourself on a waterbody that is more like a wide river than a lake. When you do, you have a picture of Claytor Lake. Claytor Lake, a 4,363 acre reservoir, stretches northeastward from Allisonia across the Pulaski County countryside for about 21 miles to its dam near Radford. From Claytor Lake State Park, visitors view a sparkling lake, bustling with boating activity, with the top of Claytor Lake dam in the distance. Visitors who want to explore can ride 15 miles upstream to Allisonia, where the New River enters the lake. Claytor Lake is shallow in areas upstream from Lighthouse Bridge, the only bridge that crosses the main lake (Pulaski County Route 672), so be cautious if you roam upstream from the bridge. Near the midpoint of Claytor Lake, the only major tributary, Peak Creek, enters the lake. If you are not familiar with Claytor Lake’s key locations, refer to the lake overview map on the last page of this report. View of Claytor Lake dam from Claytor Lake State Park’s boat ramp. American Electric Power Company (now known as Appalachian Power Company) constructed Claytor Dam in 1939 to produce hydroelectric power from the incessant flow of the New River, installing 4 hydroelectric turbines to produce electricity. Because Claytor Lake is a main stem impoundment with a large watershed upstream, water passes through more quickly than in most large Virginia reservoirs. As a result, Claytor Lake has different temperature and oxygen 1 levels than other nearby reservoirs like Smith Mountain Lake. -

Recreation & Tourism
PULASKI COUNTY is... RECREATION & TOURISM RECREATION By the numbers: Parks, public spaces and recreational activities are vital to the health and fabric of the community. They connect people, Pulaski County Owned Parks promote active living, and shape community identity. Parks and & Recreation Facilities Recreation programs have many economic benefits. Availability and easy access to quality parks and recreation enhance property values. Residents spend money during recreational The County owns and maintains nine parks with activities, which directly or indirectly helps local businesses. more than 250 acres, including: Tourism benefits from Parks and Recreation as it generates » Belsprings Park - 2 acres income from visitors. The social benefits of parks, trails and recreational facilities are clear but also hard to quantify. Parks » Draper Community Park - 6 acres create a healthy and thriving community. » Dublin Lions Club Park - 12 acres Within the County there is approximately 34,500 total acres of recreational lands. These assets are managed by local, state, » Harry Dehaven Park - 2 acres federal and private agencies such as Pulaski County, Pulaski » Loving Field - 28 acres County School Board, Town of Pulaski, Virginia Deptartment of Conservation and Recreation, Commonwealth of Virginia, U.S. » New River Community Park - 2 acres Forest Service and Boys Scouts of America. Notable natural features within the County that provide ample opportunities » Old Riverlawn Elementary Park - 13 acres for outdoor recreation are the New River and Claytor Lake. The George Washington and Jefferson National Forest lie within the » Randolph Park - 83 acres County boundaries. » Smith Farm Property - 100 acres The County desires to make significant investments on trail » Joseph L. -

An Assessment of the Bald Eagle Population Along Claytor Lake, Virginia
W&M ScholarWorks CCB Technical Reports Center for Conservation Biology (CCB) 2007 An assessment of the bald eagle population along Claytor Lake, Virginia B. D. Watts The Center for Conservation Biology, [email protected] Follow this and additional works at: https://scholarworks.wm.edu/ccb_reports Recommended Citation Watts, B. D., "An assessment of the bald eagle population along Claytor Lake, Virginia" (2007). CCB Technical Reports. 370. https://scholarworks.wm.edu/ccb_reports/370 This Report is brought to you for free and open access by the Center for Conservation Biology (CCB) at W&M ScholarWorks. It has been accepted for inclusion in CCB Technical Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. AN ASSESSMENT OF THE BALD EAGLE POPULATION ALONG CLAYTOR LAKE, VIRGINIA January, 2008 Bryan D. Watts, PhD Center for Conservation Biology College of William and Mary Williamsburg, VA 23187-8795 Recommended Citation: Watts, B. D. 2007. An assessment of the bald eagle population along Claytor Lake, Virginia. Center for Conservation Biology Technical Report Series, CCBTR-07- 12. College of William and Mary, Williamsburg, VA. 13 p. A Cooperative Project By: Normandeau Associates, Inc. & Center for Conservation Biology College of William and Mary BACKGROUND Context The United States Fish and Wildlife Service (FWS) originally listed the Bald Eagle as federally endangered on 11 March 1967 under The Endangered Species Protection Act of 1966 (16 U.S.C. 668aa-668cc) and subsequently under The Endangered Species Act of 1973 (16 U.S.C. 1531 et seq). The primary reason cited for the original listing was broad-scale population declines linked to dichloro-dephenyl- trichloroethane (DDT) and associated reproductive failure. -

Claytor Lake State Park 4400 State Park Road Dublin, VA 24084
Claytor Lake State Park 4400 State Park Road Dublin, VA 24084 Claytor Lake State Park Master Plan Executive Summary 2015 UPDATE Department of Conservation and Recreation Division of Planning and Recreation Resources 600 East Main Street, 24th Floor Richmond, Virginia 23219 Claytor Lake State Park MASTER PLAN EXECUTIVE SUMMARY 2015 UPDATE Presented for review at a public meeting on August 24, 2015; recommended for adoption by the Board of Conservation and Recreation on November 6, 2015; and reviewed for 30 days by the Virginia General Assembly. Adopted: /S/ Clyde E. Cristman, Director Department of Conservation and Recreation December 29, 2015 CLAYTOR LAKE STATE PARK MASTER PLAN EXECUTIVE SUMMARY 2015 Update This Claytor Lake State Park Master Plan Executive Summary is an update to the official unabridged master plan document adopted on March 6, 2001, then amended on August 12, 2004, by DCR Director Joseph H. Maroon. In 2010, the master plan received its five-year review and several changes were made to reflect new facilities constructed and the removal of some proposed facilities from the master plan This master plan update is intended to set forth a clear vision for the future (based on the phased development). This 2015 executive summary represents the most recent ten-year review described in §10.1-200.1 of the Code of Virginia. It outlines the desired future condition for Claytor Lake State Park when it is fully developed. Claytor Lake State Park consists of some 472 acres in Pulaski County within the New River Valley in southwest Virginia. The Blue Ridge Mountains are to the east and the Allegheny Mountains are to the west. -

Claytor Lake 2007
Claytor Lake 2007 Imagine yourself on a waterbody that is more like a wide river than a lake. When you do, you will have a picture of Claytor Lake. Claytor Lake, a 4,475-acre reservoir, stretches northeastward across the Pulaski County countryside near Radford for about 21 miles. Claytor Lake State Park visitors have a limited view of Claytor Lake. From this popular state park, visitors can view a sparkling lake, bustling with boating activity, with the top of the Claytor Lake dam in the distance. A powerboat leaving the state park boat ramp can easily reach the dam and major coves in 5 minutes. Visitors who want to explore can ride 15 miles upstream to the Allisonia boat ramp, where the New River enters the lake (up to an hour’s ride from Claytor Lake State Park). Claytor Lake is shallow in areas upstream from Lighthouse Bridge, the only bridge that crosses the main lake (Pulaski County Route 672). Near the midpoint of Claytor Lake, the only major tributary of the lake, Peak Creek, enters the lake. View of the Claytor Lake dam from Claytor Lake State Park’s boat ramp. American Electric Power Company (AEP) impounded Claytor Lake in 1939 to produce hydroelectric power from the incessant flow of the New River. Claytor Lake dam features 4 hydroelectric turbines that produce electricity. Because Claytor Lake is a main stem impoundment with a large watershed upstream, water passes through more quickly than in most large Virginia reservoirs. As a result, Claytor Lake has different temperature and oxygen levels from other nearby reservoirs like Smith Mountain Lake. -

CLAYTOR HYDROELECTRIC PROJECT FISH ENTRAINMENT and IMPINGEMENT ASSESSMENT Normandeau Associates, Inc
University of Massachusetts Amherst ScholarWorks@UMass Amherst Unpublished Works Joint EWRI-AFS Fish Passage Reference Database 2009 CLAYTOR HYDROELECTRIC PROJECT FISH ENTRAINMENT AND IMPINGEMENT ASSESSMENT Normandeau Associates, Inc. Follow this and additional works at: https://scholarworks.umass.edu/ fishpassage_unpublished_works Recommended Citation Normandeau Associates, Inc., "CLAYTOR HYDROELECTRIC PROJECT FISH ENTRAINMENT AND IMPINGEMENT ASSESSMENT" (2009). Unpublished Works. 237. Retrieved from https://scholarworks.umass.edu/fishpassage_unpublished_works/237 This Article is brought to you for free and open access by the Joint EWRI-AFS Fish Passage Reference Database at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Unpublished Works by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. CLAYTOR HYDROELECTRIC PROJECT FISH ENTRAINMENT AND IMPINGEMENT ASSESSMENT January 2009 CLAYTOR HYDROELECTRIC PROJECT FISH ENTRAINMENT AND IMPINGEMENT ASSESSMENT Prepared for APPALACHIAN POWER COMPANY Hydro Generation Department 40 Franklin Road Roanoke, VA 24011 Prepared by NORMANDEAU ASSOCIATES, INC. 1921 River Road Drumore, PA 17518 and 25 Nashua Road Bedford, NH 03110 R-20979.001 January 2009 CLAYTOR HYDRO FISH ENTRAINMENT & IMPINGEMENT ASSESSMENT Table of Contents Page 1.0 INTRODUCTION AND BACKGROUND............................................................................1 2.0 PROJECT DESCRIPTION AND GENERAL APPROACH ..............................................2