Optimising Mini-Plug Transplanting of Eucalyptus Dunnii Seedlings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
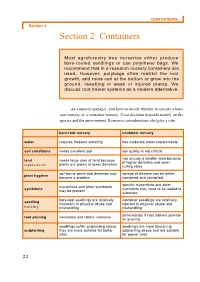
CONTAINERS Section 2 Section 2: Containers
CONTAINERS Section 2 Section 2: Containers Most agroforestry tree nurseries either produce bare-rooted seedlings or use polythene bags. We recommend that in a research nursery containers are used. However, polybags often restrict the root growth, and roots curl at the bottom or grow into the ground, resulting in weak or injured plants. We discuss root trainer systems as a modern alternative. As a nursery manager, you have to decide whether to operate a bare- root nursery or a container nursery. Your decision depends mainly on the species and the environment. Economic considerations also play a role. bare-root nursery container nursery water requires frequent watering has moderate water requirements soil conditions needs excellent soil soil quality is not critical can occupy a smaller area because land needs large area of land because of higher densities and lower plants are grown at lower densities requirements culling rates soil-borne pests and diseases may spread of disease can be better plant hygiene become a problem contained and controlled specific mycorrhiza and other mycorrhiza and other symbionts symbionts symbionts may need to be added to may be present substrate bare-root seedlings are relatively container seedlings are relatively seedling intolerant to physical abuse and tolerant to physical abuse and handling mishandling mishandling unnecessary if root trainers provide root pruning necessary and labour intensive air pruning seedlings suffer outplanting stress; seedlings are more tolerant to outplanting they are more suitable for better outplanting stress and are suitable sites for poorer sites 22 CONTAINERS Section 2 Bare-root nurseries are often recommended for on-farm nurseries because of the smaller capital investment needed. -

A Basic Guide to Tree Planting
A Basic Guide to Tree Planting Contents Planting season ................................................................................................................................................................. 3 Plant sizes and specifications for different woodland types .......................................................................................... 3 Caring for young trees before and during planting ......................................................................................................... 4 Spacing .............................................................................................................................................................................. 5 Planting for: Hedges ..................................................................................................................................................... 5 Planting for: Shelter ..................................................................................................................................................... 6 Planting Methods: ............................................................................................................................................................ 6 Planting Methods: cell grown or root trainer trees ........................................................................................................ 7 Stakes and Ties ................................................................................................................................................................ -

INFLUENCE of SOILLESS POTTING MIX and ROOT TRAINERS on GROWTH of RUBBER (Hevea Brasiliensis Muell
UNIVERSITI PUTRA MALAYSIA INFLUENCE OF SOILLESS POTTING MIX AND ROOT TRAINERS ON GROWTH OF RUBBER (Hevea brasiliensis Muell. Arg) Seedlings UPM SALISU MONSURU ADEKUNLE COPYRIGHT © FP 2017 15 INFLUENCE OF SOILLESS POTTING MIX AND ROOT TRAINERS ON GROWTH OF RUBBER (Hevea brasiliensis Muell. Arg) Seedlings UPM By SALISU MONSURU ADEKUNLE COPYRIGHT Thesis submitted to the School of Graduate Studies, Universiti Putra Malaysia, in © Fulfilment of the Requirements for the Degree of Doctor of Philosophy April 2017 COPYRIGHT All material contained within the thesis, including without limitation text, logo, icons, photographs and all other artwork, is copyright material of Universiti Putra Malaysia unless otherwise stated. Use may be made of any material contained within the thesis for non-commercial purposes from the copyright holder. Commercial use of material may only be made with the express, prior, written permission of Universiti Putra Malaysia. Copyright © Universiti Putra Malaysia UPM COPYRIGHT © DEDICATION This thesis is dedicated to my family especially my lovely wife (Maryam) and my children (Asma’ and Abu-bakr) for their kind support and sacrifice throughout my study. UPM COPYRIGHT © Abstract of thesis submitted to the Senate of Universiti Putra Malaysia in fulfillment of the requirements for the degree of Doctor of Philosophy INFLUENCE OF SOILLESS POTTING MIX AND ROOT TRAINERS ON GROWTH OF RUBBER (Hevea brasiliensis Muell. Arg) SEEDLING By SALISU MONSURU ADEKUNLE April 2017 UPM Chairman : Associate Professor Wan Mohamed Noordin Wan Daud, Dsc Faculty : Agriculture Rubber (Hevea brasiliensis) is an industrial crop that contributes significantly to the nation's economy. Nursery growers frequently report a decrease in the growth of rubber seedlings due to some negative impact of soils and other planting media. -

SWFF 2016 Semi-Annual Report Enter Date You Are First Accessing This Form 4/4/2016 Welcome Aqysta!
SWFF 2016 Semi-Annual Report Enter date you are first accessing this form 4/4/2016 Welcome aQysta! Over the following 12 survey pages you will report your aggregate progress against the milestone targets agreed upon in your award. For each aggregate number reported, please be sure to upload documentation to your Google Drive M&E folder, which has been shared with you by Stephen Simon and can be found at: http://bit.ly/reportingfolder-aqysta Please enter the email and phone number of the legally responsibe representative of your organization, in the SWFF program. * Email * Phone [email protected] +31648647260 (Netherlands), +9779808751910 (Nepal) Please answer all questions that follow reflecting cumulative progress made in the FIRST 6 MONTHS of your Year Two award. Google Drive and Form Tutorial Your "M&E" folder can be found at http://bit.ly/reportingfolder-aqysta. When you open your "M&E" folder you will see: a separate GREEN folder for each milestone a BLUE folder containing data from past program years an Excel sheet labeled "Partnerships" You will be asked to update these in specific sections of the survey. For each section of the survey, we have answered Frequently Asked Questions which can be viewed by sliding the "Show FAQ" button to the right, as in the example below. Try it! Show FAQ? >> Hidden Frequently Asked Questions Instructions for Saving and Resuming this Form Most importantly, please note that all data entered into this form is autosaved as you navigate from page to page. The "Save" button only needs to be used to generate a link to your partially-filled form, should you wish to pause and resume later. -

Teak from Sustainable Forest Management Abstract 1 2-5 December 2003, Peechi, Kerala, India
International Conference Quality Timber Products of Teak from Sustainable Forest Management Abstract 1 2-5 December 2003, Peechi, Kerala, India Teak in India: Status, Prospects and Perspectives R. P. S. Katwal Teak is a celebrated timber of the tropics and India is one of the major teak growing and utilizing countries in the world. Domestication through plantations for one and a half centuries has made teak the most widely planted and researched tropical hardwood species. Significant developments have taken place in standardizing plantation techniques, perfecting harvesting and post-harvest utilization methods and tree improvement. In spite of these efforts, teak resources of the world need immediate attention for their sustainable management. The ecological and economic aspects of raising teak in monoculture have to be considered for long-term management of supply of timber and the soils in which they are grown. Whilst bottlenecks in tree improvement and seed production have to be removed through increased basic research, the potential of frontier areas like biotechnology must also be used for rapid improvement of this plantation species. Keywords : Teak, sustainable management, improvement, productivity, conservation. R. P. S. Katwal Indian Council of Forestry Research and Education Dehra Dun - 248 006, India E-mail: [email protected] 1 International Conference Quality Timber Products of Teak from Sustainable Forest Management Abstract 2 2-5 December 2003, Peechi, Kerala, India Ups and Downs of Teak Forest Management in Indonesia Sadhardjo Siswamartana In Indonesia, teak was introduced during the fourteenth century, especially in Java, where it spread quickly in central and eastern parts. The Dutch colonial rule extensively extracted the timber, leading to degradation of teak forests in the country. -

CSTD 2016-17 Priority Theme on 'The Role of Science, Technology and Innovation in Ensuring Food Security by 2030'
INTERSESSIONAL PANEL OF THE UNITED NATIONS COMMISSION ON SCIENCE AND TECHNOLOGY FOR DEVELOPMENT (CSTD) Geneva, Switzerland 23-25 January 2017 Contribution of the United States of America to the CSTD 2016-17 priority theme on ‘The role of science, technology and innovation in ensuring food security by 2030’ DISCLAIMER: The views presented here are the contributors' and do not necessarily reflect the views and position of the United Nations or the United Nations Conference on Trade and Development. 1) Feed the Future: Advancing Food Security through Science, Technology and Innovation ( http://archive.constantcontact.com/fs182/1112173962119/archive/1115482938669.html) This is the electronic copy of USAID’s Feed the Future monthly newsletter from Oct 2013. This contains several projects with results and impact. USAID in particular highlighted the Soybean Innovation Lab (brochure attached) which is focused on four key research pillars that comprise the essential components of sustained production, improved livestock and household nutrition and sustainable market linkages for soybean development. ·Advancing Food Security through Science, Technology and Innovation ·Mobile Technology Transforming Smallholder Farmers’ Access to Fertilizer ·Disease-Resistant Cassava Increases Yields and Builds Resilience in Kenya ·Surplus in Senegal, Even Without the Rains ·136 Smallholder Farmers in Ghana Claim Drought-Index Insurance for the First Time 2) Feed the Future: Partnering for Innovation ( https://feedthefuture.gov/model/feed-future-partnering-innovation) This USAID program helps the private sector scale and market agricultural technologies for smallholder farmers through investing in technology commercialization and knowledge exchange. Through USAID Missions, the program creates and manages public-private partnerships that can improve agricultural productivity and supports the AgTechXChange to facilitate connections and knowledge sharing among entrepreneurs, investor, and nonprofit organizations. -

The Manuka & Kanuka Plantation Guide
The Mānuka & KānukaPlantation Guide April 2017 ACKNOWLEDGEMENTS PREPARATION OF THE GUIDE WAS MADE POSSIBLE THROUGH FUNDING FROM THE FOLLOWING ORGANISATIONS. WE ARE ALSO GRATEFUL TO THE INTERVIEWEES WITHOUT WHOM THIS GUIDE WOULD NOT HAVE DELIVERED THE REAL-WORLD DATA, INSIGHTS, AND INFORMATION NEEDED. THANKS ALSO TO THE MANY BOFFA MISKELL LTD STAFF WHO GENEROUSLY SHARED THEIR EXPERTISE AND COSTS INFORMATION. DOCUMENT QUALITY ASSURANCE BIBLIOGRAPHIC REFERENCE FOR CITATION: BOFFA MISKELL LIMITED 2017. THE MĀNUKA & KĀNUKA PLANTATION GUIDE: PREPARED BY: LOUISE SAUNDERS, BOFFA MISKELL LIMITED INTERVIEWS BY: MATTHEW LAY REVIEWED BY: STEPHEN FULLER, BOFFA MISKELL LIMITED DON SHEARMAN, TARANAKI DISTRICT COUNCIL GRANT BLACKIE, WAIKATO REGIONAL COUNCIL ISSUE DATE: APRIL 2017 USE AND RELIANCE THIS REPORT HAS BEEN PREPARED BY BOFFA MISKELL LIMITED ON THE BASIS OF THE INFORMATION AVAILABLE TO US AT THE TIME OF PUBLICATION. BOFFA MISKELL DOES NOT ACCEPT ANY LIABILITY OR RESPONSIBILITY IN RELATION TO THE USE OF THIS REPORT. ANY USE OR RELIANCE BY A THIRD PARTY IS AT THAT PARTY’S OWN RISK. WHERE INFORMATION HAS BEEN OBTAINED FROM OTHER EXTERNAL SOURCES, IT HAS BEEN ASSUMED THAT IT IS ACCURATE, WITHOUT INDEPENDENT VERIFICATION, UNLESS OTHERWISE INDICATED. NO LIABILITY OR RESPONSIBILITY IS ACCEPTED BY BOFFA MISKELL LIMITED FOR ANY ERRORS OR OMISSIONS. FILE REF:T15144_MANUKA PLANTING_GUIDELINE_FINAL THE MAJORITY OF THE PLATES IN THIS DOCUMENT WERE TAKEN BY LOUISE SAUNDERS (BOFFA MISKELL), OTHER THAN THE FOLLOWING: PLATE 44 - REBECCA RYDER (BOFFA MISKELL), PLATE 56 - MARCUS GIRVEN (BOFFA MISKELL), PLATES 50 - 53 AND 55 - MATTHEW LAY, AND THE IMAGES THAT APPEAR ON PAGES 26 - 31 WERE PROVIDED BY SVEN STELLIN Table of Contents 1. -

Open-Ground Indigenous Plant Nursery PROJECT REPORT March 2010
Open-Ground and Container-Raised Indigenous Plants Comparison Sustainable Farming Fund project 06-041 titled: Omaha Pilot Open-Ground Indigenous Plant Nursery PROJECT REPORT March 2010 Cimino Cole1 and David Bergin2 1Open-Ground Indigenous Plants 2Scion—a Crown research institute 590 Mahurangi West Road RD 3 Warkworth 0983 [email protected] +64 27 462 4872 +64 9 422 0872 OPEN-GROUND INDIGENOUS CONTENTS 1 Heading for Open-Ground . .. .. .. .. .. .. .. .. .. 1 2 Left to Market Forces . .. .. .. .. .. .. .. .. .. .. .. 2 3 Deforestation Catches Up With the Catchment . .. .. .. 3 4 The Search for Silver Bullets .. .. .. .. .. .. .. .. .. 4 5 Trial Transfers to Taupö . .. .. .. .. .. .. .. .. .. .. 5 6 Two and Three Times the Diameter . .. .. .. .. .. .. 6 7 A Tree Scientist’s Dream .. .. .. .. .. .. .. .. .. .. 7 8 Toetoe Gets to Play . .. .. .. .. .. .. .. .. .. .. .. 8 9 War of the Weeds . .. .. .. .. .. .. .. .. .. .. .. .. 9 10 Transplantation Shock .. .. .. .. .. .. .. .. .. .. .. 9 11 Less-Shocking Systems . .. .. .. .. .. .. .. .. .. .. 10 12 Survival at Sandspit Road . .. .. .. .. .. .. .. .. .. 11 13 Silver-Tongued Strategy . .. .. .. .. .. .. .. .. .. .. 12 14 Indigenous Forestry Strategy . .. .. .. .. .. .. .. .. 13 15 Sustainable Splash in Lake Taupö .. .. .. .. .. .. .. 14 16 Growing Trees from the Tractor Seat . .. .. .. .. .. .. 15 17 So Much for Science . .. .. .. .. .. .. .. .. .. .. .. 17 18 Bottom Line .. .. .. .. .. .. .. .. .. .. .. .. .. .. 18 Appendix: The Data . .. .. .. .. .. .. .. .. .. .. .. 19 COVER AND CONTENTS -

Faculty of Forestry
FACULTY OF FORESTRY UG degree: B. Sc. (Hons) Forestry Departments proposed by the V Deans Committee: 1. Silviculture and Agroforestry (SAF) 2. Forest Biology and Tree Improvement (FBT) 3. Natural Resource Management (NRM) 4. Forest Product Utilization (FPU) 5. Wildlife Sciences (WLS) 6. Social and Basic Sciences (SBS) DEPARTMENTWISE DISTRIBUTION OF COURSES Course Code Discipline/Course title Credit Hrs Silviculture and Agroforestry (SAF) SAF 111 Introduction to Forestry 2(2+0) SAF 112 Dendrology 3(2+1) SAF 121 Theory and Practice of Silviculture 3(2+1) SAF 211 Principles of Agroforestry 3(2+1) SAF 212 Forest Mensuration 3(2+1) SAF 221 Forest Management 3(2+1) SAF 222 Silviculture of Indian Trees 3(2+1) SAF 311 Forest Hydrology and Watershed Management 3(2+1) SAF 321 Plantation Forestry 3(2+1) FEW 411 Forestry Work Experience (Student READY, FOWE) 20(0+20) SAF 421 Agroforestry Systems and Management 2(2+0) FEL Experiential Learning -I 5(0+5) FEL Experiential Learning -II 5(0+5) PWD Project Work & Dissertation 10(0+10) Total 20+48=68 Forest Biology and Tree Improvement (FBT) FBT 121 Plant Physiology 3(2+1) FBT 122 Plant Cytology and Genetics 2(1+1) FBT 211 Tree Improvement 3(2+1) FBT 221 Seed Technology & Nursery Management 3(2+1) FEW 411 Forestry Work Experience (Student READY, FOWE) 20(0+20) FBT 421 Forest Biotechnology 3(2+1) FEL Experiential Learning 5(0+5) FEL Experiential Learning 5(0+5) PWD Project Work & Dissertation 10(0+10) Total 9+45=54 Natural Resource Management (NRM) NRM 111 Introduction to Agronomy and Horticulture 3(2+1) -

Studies on Refinement of Container Size and Potting Mixture for Production of Quality Seedlings in Spruce (Picea Smithiana Wall
Open Journal of Forestry, 2015, 5, 733-739 Published Online October 2015 in SciRes. http://www.scirp.org/journal/ojf http://dx.doi.org/10.4236/ojf.2015.57065 Studies on Refinement of Container Size and Potting Mixture for Production of Quality Seedlings in Spruce (Picea Smithiana Wall. Boiss) J. A. Mugloo, P. A. Khan, A. H. Mughal*, K. N. Qasir, S. N. Zaffar, Gowher Parrey Faculty of Forestry, SKUAST, Kashmir Email: [email protected], [email protected], *[email protected], [email protected], [email protected], [email protected] Received 28 June 2014; accepted 5 October 2015; published 8 October 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Spruce (Picea Smithiana) is an evergreen tree and distributed through the temperate Himalaya at the elevation of 2000 - 3500 m. Natural regeneration of spruce (Picea smithiana) is generally slow and almost negligible due to a number of factors e.g. presence of un decomposed raw humus on forest floor low germinative capacity of seed and infrequent good seed years. In order to supple- ment natural regeneration through artificial means, nursery raising assumes significance. The present study aimed at optimizing the root trainer container size and the potting mixture ingre- dients and their proportion for the production of quality nursery stock of Picea smithiana. Nine combinations of potting mixture ingredients (Forest Soil, Garden Soil, Sand, FYM and Lake weed) were tested in various ratios for the optimization of potty mixture in different root trainer sizes. -

Improvement of Seedling Production System in Forestry Sector and Its Impact on Seedling Health
Working Papers of the Finnish Forest Research Institute 11 http://www.metla.fi/julkaisut/workingpapers/2005/mwp011.htm Improvement of seedling production system in forestry sector and its impact on seedling health C. Mohanan and J.K. Sharma Kerala Forest Research Institute, Peechi 680 653, Kerala India More than 120 forestry species are being utilized in India for various afforestation programmes. Among these, teak (Tectona grandis), eucalypts (Eucalyptus grandis, E. tereticornis), acacias (Acacia auriculiformis, A. mangium), and poplars (Populus spp. are exploited on a large-scale and the annual planting rate is about 1.78 Mha. Production of planting stock and stand establishment are affected by various limiting factors, of which diseases play a major role. The introduction of root trainer technology has made a tremendous impact on forest nursery seedling production. The technology offers production of large number of healthy, uniform-sized planting stock within 90 days. Using the technology also improves the quality of seedlings and clonal plants since it allows for growers to better manipulate mycorrhizae, biofertilizers, biopesticides and micronutrients. The other advantage over seedbed and polythene container nurseries is that root spiraling of seedlings can be avoided. However, the technology needs further optimization and growing media have to be developed and standardized depending on the seedling crop, local climatic conditions, and the planting technique to be followed. This paper highlights the modernization of planting stock production system and its impact on nursery management. Introduction Even though, forest tree seedling production systems has been revolutionized in many countries, in India, production of planting stock is still largely depended upon conventional methods. -

Guidelines to Produce Quality Planting Material of Agroforestry Species
Quality Planting Material Production Guidelines Guidelines to Produce Quality Planting Material of Agroforestry Species 1 S u c CAFRI Quality Planting Materialc Production Guidelines The Indian Council of Agricultue ral Research (ICAR) established Central s Agroforestry Research Institute s (CAFRI), erstwhile known as the National f Research Centre for Agroforestryu (NRCAF), at Jhansi on 8 May 1988 to cater to basic, strategic and applied resl earch needs in the field of agroforestry. The Institute coordinates a large agroA forestry network—the All India Coordinated Research Project on Agroforesg try—with 37 centres located in different r agro-climates of the country. o f o r ICAR e The Indian Council of Agricultus ral Research (ICAR) is an autonomous t organization under the Departmer nt of Agricultural Research and Education (DARE), Ministry of Agriculture ay nd Farmers Welfare, Government of India. ICAR, established on 16 July 19M 29 with headquarters at New Delhi, is the apex body for coordinating, guidino g and managing research and education in d agriculture including horticulture, fe isheries and animal sciences in the country. With 101 ICAR institutes and 71l agricultural universities spread across the s country this is one of the largest na tional agricultural systems in the world. The f ICAR has played a pioneering o role in ushering in Green Revolution and r subsequent developments in ag riculture in India through its research and technology development; and hD as actively contributed in formulation and i f implementation of agroforestry pof licy and mission. e r ICRAF e World Agroforestry (ICRAF) is n a centre of science and development t excellence that harnesses theA benefits of trees for people and the environment.