Menopause-PPT-Physiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification and Pharmacology of Progestins
Maturitas 46S1 (2003) S7–S16 Classification and pharmacology of progestins Adolf E. Schindler a,∗, Carlo Campagnoli b, René Druckmann c, Johannes Huber d, Jorge R. Pasqualini e, Karl W. Schweppe f, Jos H. H. Thijssen g a Institut für Medizinische Forschung und Fortbildung, Universitätsklinikum, Hufelandstr. 55, Essen 45147, Germany b Ospedale Ginecologico St. Anna, Corso Spezia 60, 10126 Torino, Italy c Ameno-Menopause-Center, 12, Rue de France, 06000 Nice, France d Abt. für Gynäkologische Endokrinologie, AKH Wien, Währingergürtel 18-20, 1090 Wien, Austria e Institute de Puériculture26, Boulevard Brune, 75014 Paris, France f Abt. für Gynäkologie und Geburtshilfe, Ammerland Klinik, Langestr.38, 26622 Westerstede, Germany g Department of Endocrinology, Universitair Medisch Centrum Utrecht, P.O. Box 85090, 3508 AB Utrecht, The Netherlands Abstract Besides the natural progestin, progesterone, there are different classes of progestins, such as retroprogesterone (i.e. dydroges- terone), progesterone derivatives (i.e. medrogestone) 17␣-hydroxyprogesterone derivatives (i.e. chlormadinone acetate, cypro- terone acetate, medroxyprogesterone acetate, megestrol acetate), 19-norprogesterone derivatives (i.e. nomegestrol, promege- stone, trimegestone, nesterone), 19-nortestosterone derivatives norethisterone (NET), lynestrenol, levonorgestrel, desogestrel, gestodene, norgestimate, dienogest) and spironolactone derivatives (i.e. drospirenone). Some of the synthetic progestins are prodrugs, which need to be metabolized to become active compounds. Besides -

Safety Data Sheet
SAFETY DATA SHEET SECTION 1: PRODUCT IDENTIFICATION PRODUCT NAME DHEA (Prasterone) (Micronized) PRODUCT CODE 0733 SUPPLIER MEDISCA Inc. Tel.: 1.800.932.1039 | Fax.: 1.855.850.5855 661 Route 3, Unit C, Plattsburgh, NY, 12901 3955 W. Mesa Vista Ave., Unit A-10, Las Vegas, NV, 89118 6641 N. Belt Line Road, Suite 130, Irving, TX, 75063 MEDISCA Pharmaceutique Inc. Tel.: 1.800.665.6334 | Fax.: 514.338.1693 4509 Rue Dobrin, St. Laurent, QC, H4R 2L8 21300 Gordon Way, Unit 153/158, Richmond, BC V6W 1M2 MEDISCA Australia PTY LTD Tel.: 1.300.786.392 | Fax.: 61.2.9700.9047 Unit 7, Heritage Business Park 5-9 Ricketty Street, Mascot, NSW 2020 EMERGENCY PHONE CHEMTREC Day or Night Within USA and Canada: 1-800-424-9300 NSW Poisons Information Centre: 131 126 USES Adjuvant; Androgen SECTION 2: HAZARDS IDENTIFICATION GHS CLASSIFICATION Toxic to Reproduction (Category 2) PICTOGRAM SIGNAL WORD Warning HAZARD STATEMENT(S) Reproductive effector, prohormone. Suspected of damaging fertility or the unborn child. May cause harm to breast-fed children. Causes serious eye irritation. Causes skin and respiratory irritation. Very toxic to aquatic life with long lasting effects. AUSTRALIA-ONLY HAZARDS Not Applicable. PRECAUTIONARY STATEMENT(S) Prevention Wash thoroughly after handling. Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Do not breathe dusts or mists. Do not eat, drink or smoke when using this product. Avoid contact during pregnancy/while nursing. Wear protective gloves, protective clothing, eye protection, face protection. Avoid release to the environment. Response IF ON SKIN (HAIR): Wash with plenty of water. -

2011/097571 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau „ (10) International Publication Number (43) International Publication Date \i\ 11 August 2011 (11.08.2011) 2011/097571 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 38/22 (2006.01) A61P 11/06 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/573 (2006.01) A61P 11/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61P 29/00 (2006.01) A61P 37/00 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, PCT/US201 1/023917 KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (22) International Filing Date: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 7 February 201 1 (07.02.201 1) NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (25) Filing Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/302,325 8 February 2010 (08.02.2010) US GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, 13/021,950 7 February 201 1 (07.02.201 1) US ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (71) Applicant (for all designated States except US): EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, PRAIRIE PHARMACEUTICALS, LLC [US/US]; LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, 1041 1 Motor City Drive, Suite 750, Bethesda, MD 20817 SM, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, (US). -

Vargas KEA, Et Al. Hepatotoxicity Associated with Methylstenbolone and Copyright© Vargas KEA, Et Al
1. Medical Journal of Clinical Trials & Case Studies ISSN: 2578-4838 Hepatotoxicity Associated with Methylstenbolone and Stanozolol Abuse Vargas KEA*, Guaraná TA, Biccas BN, Agoglia LV, Carvalho ACG, Case Report Gismondi R and Esberard EBC Volume 2 Issue 5 Received Date: July 27, 2018 Department of Gastroenterology/Hepatology, Department of Clinical Medicine, and Published Date: September 03, 2018 Department of Pathology, Antônio Pedro University Hospital, Federal Fluminense DOI: 10.23880/mjccs-16000176 University, Rio de Janeiro, Brazil *Corresponding author: Vargas Karen Elizabeth Arce, Department of Gastroenterology/Hepatology, Department of Clinical Medicine, and Department of Pathology, Antônio Pedro University Hospital, Federal Fluminense University, Rio de Janeiro, Ernani do Amaral Peixoto Avenue, 935. Ap.901 / Cep.24020043, Brazil, Tel: 005521981584624; Email: [email protected] Abstract Background & Objectives: Drug hepatotoxicity is a major cause of liver disease. Many drugs are well known to induce liver damage. Some toxic products, like anabolic androgenic steroids, that are pharmaceutical preparations since they contain pharmaceutically active substance, are available as nutritional supplements. Many patients are used to consume these like dietary stuff. Methods: We introduce a case series of two patients who developed hepatic damage after the consumption of anabolic- androgenic steroids, accompanied by a detailed bibliographic research on this topic. Results: We present two young men who developed significant liver damage, both with hyperbilirubinemia pattern after consumption of anabolic-androgenic steroids. This was associated with considerable morbidity, although both recovered without liver transplantation. The two anabolic-androgenic steroids were being marketed as dietary supplements. Conclusions: Although not well controlled substances in Brazil, anabolic-androgenic steroids are cause of severe hepatotoxicity. -

Combined Estrogen–Progestogen Menopausal Therapy
COMBINED ESTROGEN–PROGESTOGEN MENOPAUSAL THERAPY Combined estrogen–progestogen menopausal therapy was considered by previous IARC Working Groups in 1998 and 2005 (IARC, 1999, 2007). Since that time, new data have become available, these have been incorporated into the Monograph, and taken into consideration in the present evaluation. 1. Exposure Data 1.1.2 Progestogens (a) Chlormadinone acetate Combined estrogen–progestogen meno- Chem. Abstr. Serv. Reg. No.: 302-22-7 pausal therapy involves the co-administration Chem. Abstr. Name: 17-(Acetyloxy)-6-chlo- of an estrogen and a progestogen to peri- or ropregna-4,6-diene-3,20-dione menopausal women. The use of estrogens with IUPAC Systematic Name: 6-Chloro-17-hy- progestogens has been recommended to prevent droxypregna-4,6-diene-3,20-dione, acetate the estrogen-associated risk of endometrial Synonyms: 17α-Acetoxy-6-chloro-4,6- cancer. Evidence from the Women’s Health pregnadiene-3,20-dione; 6-chloro-Δ6-17- Initiative (WHI) of adverse effects from the use acetoxyprogesterone; 6-chloro-Δ6-[17α] of a continuous combined estrogen–progestogen acetoxyprogesterone has affected prescribing. Patterns of exposure Structural and molecular formulae, and relative are also changing rapidly as the use of hormonal molecular mass therapy declines, the indications are restricted, O CH and the duration of the therapy is reduced (IARC, 3 C 2007). CH3 CH3 O C 1.1 Identification of the agents CH3 H O 1.1.1 Estrogens HH For Estrogens, see the Monograph on O Estrogen-only Menopausal Therapy in this Cl volume. C23H29ClO4 Relative molecular mass: 404.9 249 IARC MONOGRAPHS – 100A (b) Cyproterone acetate Structural and molecular formulae, and relative Chem. -

Records of Pharmaceutical and Biomedical Sciences
REVIEW ARTICLE RECORDS OF PHARMACEUTICAL AND BIOMEDICAL SCIENCES Effect of Exogenous Anabolic Androgenic Steroids on Testosterone/ Epitestosterone Ratio and its Application on Athlete Biological Passport in Egypt Hanem A. Khalil a, Dina M. Abo-Elmatty b, Rosa V. Alemany c, Noha M. Mesbah b a Egyptian Anti-Doping Organization, Cairo, Egypt. b Faculty of Pharmacy, Department of Biochemistry Suez Canal University, Ismailia, Egypt. C Catalonian Anti-Doping Laboratory of Fundacio IMIM, Barcelona, Spain. Abstract Received on: 01.09. 2018 Using the Anabolic Androgenic Steroid (AAS) agents is evident not only Revised on: 21. 10. 2018 within the competitive senior and junior athletes, but also in non-sporting contexts by individuals seeking to „improve‟ their physique. No accurate data Accepted on: 01. 11. 2018 is available for the prevalence of AAS misuse among athletes. Studies suggest that it may be 1–5% of the population; with the prevalence being higher in males. Many studies documented side effects and health hazards with the misuse of anabolic steroids, where these were accused as a cause of Correspondence Author: deaths among athletes. Intake of exogenous anabolic steroids disturbed the Testosterone / Epitestosterone (T/E) ratio causing its evaluation above the Tel:+201270206648. normal level. This review outlines the anabolic steroids, its side effects and E-mail address: health impacts in both the sporting and physique development contexts. It also provides a brief review of the history of AAS as doping agents and [email protected] athlete biological passport. Conclusion: Doping among athletes is a widespread public health and social problem. Many studies have shown that both short- and long-term health complications have consequences and dependencies. -

Combined Estrogen–Progestogen Menopausal Therapy
PHARMACEUTICALS volume 100 A A review of humAn cArcinogens This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon, 14-21 October 2008 LYON, FRANCE - 2012 iArc monogrAphs on the evAluAtion of cArcinogenic risks to humAns COMBINED ESTROGEN–PROGESTOGEN MENOPAUSAL THERAPY Combined estrogen–progestogen menopausal therapy was considered by previous IARC Working Groups in 1998 and 2005 (IARC, 1999, 2007). Since that time, new data have become available, these have been incorporated into the Monograph, and taken into consideration in the present evaluation. 1. Exposure Data 1.1.2 Progestogens (a) Chlormadinone acetate Combined estrogen–progestogen meno- Chem. Abstr. Serv. Reg. No.: 302-22-7 pausal therapy involves the co-administration Chem. Abstr. Name: 17-(Acetyloxy)-6-chlo- of an estrogen and a progestogen to peri- or ropregna-4,6-diene-3,20-dione menopausal women. The use of estrogens with IUPAC Systematic Name: 6-Chloro-17-hy- progestogens has been recommended to prevent droxypregna-4,6-diene-3,20-dione, acetate the estrogen-associated risk of endometrial Synonyms: 17α-Acetoxy-6-chloro-4,6- cancer. Evidence from the Women’s Health pregnadiene-3,20-dione; 6-chloro-Δ6-17- Initiative (WHI) of adverse effects from the use acetoxyprogesterone; 6-chloro-Δ6-[17α] of a continuous combined estrogen–progestogen acetoxyprogesterone has affected prescribing. Patterns of exposure Structural and molecular formulae, and relative are also changing rapidly as the use of hormonal molecular mass therapy declines, the indications are restricted, O CH and the duration of the therapy is reduced (IARC, 3 C 2007). -

Reproductive DHEA
Reproductive DHEA Analyte Information - 1 - DHEA Introduction DHEA (dehydroepiandrosterone), together with other important steroid hormones such as testosterone, DHT (dihydrotestosterone) and androstenedione, belongs to the group of androgens. Androgens are a group of C19 steroids that stimulate or control the development and maintenance of male characteristics. This includes the activity of the male sex organs and the development of secondary sex characteristics. Androgens are also precursors of all estrogens, the female sex hormones. DHEA (dehydroepiandrosterone) is the aromatic C19-steroid composed of a 10,13-dimethyl, 3-hydroxy group and 17-ketone. Its chemical name is 3β-hydroxy-5-androsten-17-one, its summary formula is C19H28O2, and its molecular weight (Mr) is 288.4 Da. The structural formulas of DHEA and related androgens are shown in Fig.1 Fig.1: Structural formulas of the most important androgens DHEA Androstenedione Testosterone Dihydrotestosterone There are more than 40 other names used for DHEA, including: (+)-Dehydroisoandrosterone; (3beta, 16alpha)-3,16-dihydroxy-androst-5-en- 17-one; 5,6-Dehydroisoandrosterone; 17-Chetovis, 17-Hormoforin, Andrestenol, Diandron, Prasterone and so on. As DHEA is very closely connected with its sulfate form DHEA-S, both hormones are mentioned together in the following text. Biosynthesis DHEA is the steroid hormone belonging to the weak androgens. DHEA and DHEA-S are the major C19 steroids produced from cholesterol by the zona reticularis of the adrenal cortex (Fig.2). DHEA is also produced in small quantities in the gonads (testis and ovary3,8,14), in adipose tissue and in the brain. From this point of view DHEA belongs to the neurosteroids22. -

Steroids and Other Appearance and Performance Enhancing Drugs (Apeds) Research Report
Research Report Revised Febrero 2018 Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Table of Contents Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Introduction What are the different types of APEDs? What is the history of anabolic steroid use? Who uses anabolic steroids? Why are anabolic steroids misused? How are anabolic steroids used? What are the side effects of anabolic steroid misuse? How does anabolic steroid misuse affect behavior? What are the risks of anabolic steroid use in teens? How do anabolic steroids work in the brain? Are anabolic steroids addictive? How are anabolic steroids tested in athletes? What can be done to prevent steroid misuse? What treatments are effective for anabolic steroid misuse? Where can I get further information about steroids? References Page 1 Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Esta publicación está disponible para su uso y puede ser reproducida, en su totalidad, sin pedir autorización al NIDA. Se agradece la citación de la fuente, de la siguiente manera: Fuente: Instituto Nacional sobre el Abuso de Drogas; Institutos Nacionales de la Salud; Departamento de Salud y Servicios Humanos de los Estados Unidos. Introduction Appearance and performance enhancing drugs (APEDs) are most often used by males to improve appearance by building muscle mass or to enhance athletic performance. Although they may directly and indirectly have effects on a user’s mood, they do not produce a euphoric high, which makes APEDs distinct from other drugs such as cocaine, heroin, and marijuana. However, users may develop a substance use disorder, defined as continued use despite adverse consequences. -

Michigan Department of Community Health
Michigan Department 0f Community Health, Office of Drug Control Policy The Michigan Association of Community Mental Health March 27, 2009 NationalNational TrendsTrends andand DeterrentDeterrent StrategiesStrategies ForFor PrescriptionPrescription andand OTCOTC DrugDrug AbuseAbuse Joseph Rannazzisi Deputy Assistant Administrator Office of Diversion Control Drug Enforcement Administration IntroductionIntroduction BackgroundBackground andand StatisticsStatistics RegulatoryRegulatory ControlControl MethodsMethods ofof DiversionDiversion InternetInternet DiversionDiversion CommonlyCommonly DivertedDiverted PharmaceuticalsPharmaceuticals Steroids/Steroids/hGHhGH DietaryDietary SupplementsSupplements SalviaSalvia DivinorumDivinorum TheThe 19601960’’ss Marijuana Seconal LSD Dexedrine Meprobamate TheThe 19701970’’ss Heroin T’s and Blues 4’s and Doors (Talwin and Pyrabenzamine) TheThe 19801980’’ss Tylenol w/Codeine and Doriden Hydromorphone Cocaine TheThe 19901990’’ss Oxycodone Methamphetamine 20002000 Hydrocodone Ketamine Alprazolam Flunitrazapam MDMA (Rohypnol) Scope and Extent of Problem 2004 2007 0.30.3 millionmillion 0.35 million SedativesSedatives 1.21.2 millionmillion 1.11.1 millionmillion StimulantsStimulants 1.61.6 millionmillion 1.81.8 millionmillion Anti-Anxiety Medication 4.44.4 millionmillion 5.25.2 millionmillion NarcoticNarcotic PainPain RelieversRelievers Source: 2004 and 2007 National Survey on Drug Use and Health TeensTeens andand TheirTheir AttitudesAttitudes 1 in 5 teens report abusing Rx medications to get high 2 in -

The Treatment of Monophasic Cycles with the Retroprogesterone Ro 4-8347
The treatment of monophasic cycles with the retroprogesterone Ro 4-8347 Autor(en): Taubert, H.-D. / Jürgensen, O. Objekttyp: Article Zeitschrift: Bulletin der Schweizerischen Akademie der Medizinischen Wissenschaften = Bulletin de l'Académie Suisse des Sciences Medicales = Bollettino dell' Accademia Svizzera delle Scienze Mediche Band (Jahr): 25 (1969) PDF erstellt am: 25.09.2021 Persistenter Link: http://doi.org/10.5169/seals-307794 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das Fehlen von Informationen. Dies gilt auch für Inhalte Dritter, die über dieses Angebot zugänglich sind. Ein Dienst der ETH-Bibliothek ETH Zürich, Rämistrasse 101, 8092 Zürich, Schweiz, www.library.ethz.ch http://www.e-periodica.ch Abteilung fiir gynäkologische Endokrinologie (Leiter: Prof. TT.-T). Taubert) der Universitälsfraueiiklinik, Frankfurt/Main (Direktor; Prof. 0. Käser) The Treatment of Monophasic Cycles with the Retroprogesterone Ro 4-8347 H.-l). -
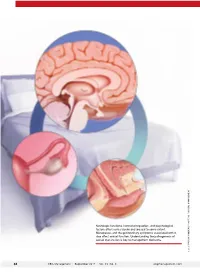
Neurologic Functions, Hormonal Regulation, and Psychological Factors Affect Sexual Desire and Arousal to Some Extent
Neurologic functions, hormonal regulation, and psychological factors affect sexual desire and arousal to some extent. Menopause, and the genitourinary symptoms associated with it, also affect sexual function. Understanding the pathogenesis of sexual dysfunction is key to management decisions. ILLUSTRATION: KIMBERLY MARTENS FOR OBG MANAGEMENT MARTENS KIMBERLY ILLUSTRATION: 34 OBG Management | September 2017 | Vol. 29 No. 9 obgmanagement.com UPDATE FEMALE SEXUAL DYSFUNCTION New and emerging treatment options hold promise for improving outcomes in this undertreated disorder ❯❯ Barbara S. Levy, MD Dr. Levy is Vice President for Health Policy at the American College of Obstetricians and Gynecologists, Washington, DC. The author reports no financial relationships relevant to this article. exual function is a complex, multifac- chronic conditions such as diabetes and S eted process mediated by neurologic back pain.4 functions, hormonal regulation, and psy- Understanding the pathogenesis of chological factors. What could possibly female sexual dysfunction helps to guide go wrong? our approach to its management. Indeed, IN THIS As it turns out, quite a lot. Female sex- increased understanding of its pathology has ARTICLE ual dysfunction is a common, vastly under- helped to usher in new and emerging treat- treated sexual health problem that can have ment options, as well as a personalized, bio- How hormones, wide-reaching effects on a woman’s life. psychosocial approach to its management. experience, These effects may include impaired body In this Update, I discuss the interplay of and behavior affect image, self-confidence, and self-worth. Sex- physiologic and psychological factors that the brain ual dysfunction also can contribute to rela- affect female sexual function as well as the page 36 tionship dissatisfaction and leave one feeling latest options for its management.