DNA Methylation Alterations&Mdash
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RNA-Seq Transcriptome Analysis Of
on: Sequ ati en er c Le Luyer et al, Next Generat Sequenc & Applic 2015, 2:1 n in e g G & t x A DOI: 10.4172/2469-9853.1000112 e p Journal of p N l f i c o a l t a i o n r ISSN: 2469-9853n u s o J Next Generation Sequencing & Applications Research Article Open Access RNA-Seq Transcriptome Analysis of Pronounced Biconcave Vertebrae: A Common Abnormality in Rainbow Trout (Oncorhynchus mykiss, Walbaum) Fed a Low-Phosphorus Diet Le Luyer J1, Deschamps MH1, Proulx E1, Poirier Stewart N1, Droit A2, Sire JY3, Robert C1 and Vandenberg GW1* 1Département of des sciences animales, Pavillon Paul-Comtois, Université Laval, Québec, QC, Canada G1V 0A6, Canada 2Department of Molecular Medicine, Centre de Recherche du CHU de Québec, Université Laval, Québec, QC, G1V 4G2 Canada 3Institut de Biologie Paris-Seine, UMR 7138-Evolution Paris-Seine, Université Pierre et Marie Curie, Paris, France Abstract The prevalence of bone deformities, particularly linked with mineral deficiency, is an important issue for fish production. Juvenile triploid rainbow trout (Oncorhynchus mykiss) were fed a low-phosphorus (P) diet for 27 weeks (60 to 630 g body mass). At study termination, 24.9% of the fish fed the low-P diet displayed homogeneous biconcave vertebrae (deformed vertebrae phenotype), while 5.5% displayed normal vertebral phenotypes for the entire experiment. The aim of our study was to characterize the deformed phenotype and identify the putative genes involved in the appearance of P deficiency-induced deformities. Both P status and biomechanical measurements showed that deformed vertebrae were significantly less mineralized (55.0 ± 0.4 and 59.4 ± 0.5,% ash DM, for deformed and normal vertebrae, respectively) resulting in a lower stiffness (80.3 ± 9.0 and 140.2 ± 6.3 N/mm, for deformed and normal phenotypes, respectively). -

Appendix 2. Significantly Differentially Regulated Genes in Term Compared with Second Trimester Amniotic Fluid Supernatant
Appendix 2. Significantly Differentially Regulated Genes in Term Compared With Second Trimester Amniotic Fluid Supernatant Fold Change in term vs second trimester Amniotic Affymetrix Duplicate Fluid Probe ID probes Symbol Entrez Gene Name 1019.9 217059_at D MUC7 mucin 7, secreted 424.5 211735_x_at D SFTPC surfactant protein C 416.2 206835_at STATH statherin 363.4 214387_x_at D SFTPC surfactant protein C 295.5 205982_x_at D SFTPC surfactant protein C 288.7 1553454_at RPTN repetin solute carrier family 34 (sodium 251.3 204124_at SLC34A2 phosphate), member 2 238.9 206786_at HTN3 histatin 3 161.5 220191_at GKN1 gastrokine 1 152.7 223678_s_at D SFTPA2 surfactant protein A2 130.9 207430_s_at D MSMB microseminoprotein, beta- 99.0 214199_at SFTPD surfactant protein D major histocompatibility complex, class II, 96.5 210982_s_at D HLA-DRA DR alpha 96.5 221133_s_at D CLDN18 claudin 18 94.4 238222_at GKN2 gastrokine 2 93.7 1557961_s_at D LOC100127983 uncharacterized LOC100127983 93.1 229584_at LRRK2 leucine-rich repeat kinase 2 HOXD cluster antisense RNA 1 (non- 88.6 242042_s_at D HOXD-AS1 protein coding) 86.0 205569_at LAMP3 lysosomal-associated membrane protein 3 85.4 232698_at BPIFB2 BPI fold containing family B, member 2 84.4 205979_at SCGB2A1 secretoglobin, family 2A, member 1 84.3 230469_at RTKN2 rhotekin 2 82.2 204130_at HSD11B2 hydroxysteroid (11-beta) dehydrogenase 2 81.9 222242_s_at KLK5 kallikrein-related peptidase 5 77.0 237281_at AKAP14 A kinase (PRKA) anchor protein 14 76.7 1553602_at MUCL1 mucin-like 1 76.3 216359_at D MUC7 mucin 7, -

Identification of Novel CD4+ T Cell Subsets in the Target Tissue Of
Identification of Novel CD4+ T Cell Subsets in the Target Tissue of Sjögren's Syndrome and Their Differential Regulation by the Lymphotoxin/LIGHT Signaling Axis This information is current as of October 2, 2021. Scott Haskett, Jian Ding, Wei Zhang, Alice Thai, Patrick Cullen, Shanqin Xu, Britta Petersen, Galina Kuznetsov, Luke Jandreski, Stefan Hamann, Taylor L. Reynolds, Norm Allaire, Timothy S. Zheng and Michael Mingueneau J Immunol 2016; 197:3806-3819; Prepublished online 7 Downloaded from October 2016; doi: 10.4049/jimmunol.1600407 http://www.jimmunol.org/content/197/10/3806 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2016/10/06/jimmunol.160040 Material 7.DCSupplemental References This article cites 46 articles, 8 of which you can access for free at: http://www.jimmunol.org/content/197/10/3806.full#ref-list-1 Why The JI? Submit online. by guest on October 2, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. -
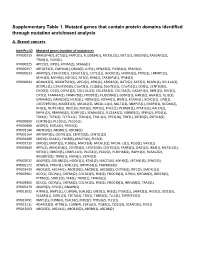
Supplementary Table 1. Mutated Genes That Contain Protein Domains Identified Through Mutation Enrichment Analysis
Supplementary Table 1. Mutated genes that contain protein domains identified through mutation enrichment analysis A. Breast cancers InterPro ID Mutated genes (number of mutations) IPR000219 ARHGEF4(2), ECT2(1), FARP1(1), FLJ20184(1), MCF2L2(1), NET1(1), OBSCN(5), RASGRF2(2), TRAD(1), VAV3(1) IPR000225 APC2(2), JUP(1), KPNA5(2), SPAG6(1) IPR000357 ARFGEF2(2), CMYA4(1), DRIM(2), JUP(1), KPNA5(2), PIK3R4(1), SPAG6(1) IPR000533 AKAP9(2), C10orf39(1), C20orf23(1), CUTL1(1), HOOK1(1), HOOK3(1), KTN1(2), LRRFIP1(3), MYH1(3), MYH9(2), NEF3(1), NF2(1), RSN(1), TAX1BP1(1), TPM4(1) IPR000694 ADAM12(3), ADAMTS19(1), APC2(2), APXL(1), ARID1B(1), BAT2(2), BAT3(1), BCAR1(1), BCL11A(2), BCORL1(1), C14orf155(3), C1orf2(1), C1QB(1), C6orf31(1), C7orf11(1), CD2(1), CENTD3(3), CHD5(3), CIC(3), CMYA1(2), COL11A1(3), COL19A1(2), COL7A1(3), DAZAP1(1), DBN1(3), DVL3(1), EIF5(1), FAM44A(1), FAM47B(1), FHOD1(1), FLJ20584(1), G3BP2(2), GAB1(2), GGA3(1), GLI1(3), GPNMB(2), GRIN2D(3), HCN3(1), HOXA3(2), HOXA4(1), IRS4(1), KCNA5(1), KCNC2(1), LIP8(1), LOC374955(1), MAGEE1(2), MICAL1(2), MICAL‐L1(1), MLLT2(1), MMP15(1), N4BP2(1), NCOA6(2), NHS(1), NUP214(3), ODZ1(3), PER1(2), PER2(1), PHC1(1), PLXNB1(1), PPM1E(2), RAI17(2), RAPH1(2), RBAF600(2), SCARF2(1), SEMA4G(1), SLC16A2(1), SORBS1(1), SPEN(2), SPG4(1), TBX1(1), TCF1(2), TCF7L1(1), TESK1(1), THG‐1(1), TP53(18), TRIF(1), ZBTB3(2), ZNF318(2) IPR000909 CENTB1(2), PLCB1(1), PLCG1(1) IPR000998 AEGP(3), EGFL6(2), PRSS7(1) IPR001140 ABCB10(2), ABCB6(1), ABCB8(2) IPR001164 ARFGAP3(1), CENTB1(2), CENTD3(3), CENTG1(2) IPR001589 -

Epigenetic Mechanisms Are Involved in the Oncogenic Properties of ZNF518B in Colorectal Cancer
Epigenetic mechanisms are involved in the oncogenic properties of ZNF518B in colorectal cancer Francisco Gimeno-Valiente, Ángela L. Riffo-Campos, Luis Torres, Noelia Tarazona, Valentina Gambardella, Andrés Cervantes, Gerardo López-Rodas, Luis Franco and Josefa Castillo SUPPLEMENTARY METHODS 1. Selection of genomic sequences for ChIP analysis To select the sequences for ChIP analysis in the five putative target genes, namely, PADI3, ZDHHC2, RGS4, EFNA5 and KAT2B, the genomic region corresponding to the gene was downloaded from Ensembl. Then, zoom was applied to see in detail the promoter, enhancers and regulatory sequences. The details for HCT116 cells were then recovered and the target sequences for factor binding examined. Obviously, there are not data for ZNF518B, but special attention was paid to the target sequences of other zinc-finger containing factors. Finally, the regions that may putatively bind ZNF518B were selected and primers defining amplicons spanning such sequences were searched out. Supplementary Figure S3 gives the location of the amplicons used in each gene. 2. Obtaining the raw data and generating the BAM files for in silico analysis of the effects of EHMT2 and EZH2 silencing The data of siEZH2 (SRR6384524), siG9a (SRR6384526) and siNon-target (SRR6384521) in HCT116 cell line, were downloaded from SRA (Bioproject PRJNA422822, https://www.ncbi. nlm.nih.gov/bioproject/), using SRA-tolkit (https://ncbi.github.io/sra-tools/). All data correspond to RNAseq single end. doBasics = TRUE doAll = FALSE $ fastq-dump -I --split-files SRR6384524 Data quality was checked using the software fastqc (https://www.bioinformatics.babraham. ac.uk /projects/fastqc/). The first low quality removing nucleotides were removed using FASTX- Toolkit (http://hannonlab.cshl.edu/fastxtoolkit/). -

Integrated Transcriptomic, Phenotypic, and Functional Study Reveals Tissue-Specific Immune Properties of Mesenchymal Stromal
Integrated Transcriptomic, Phenotypic, and Functional Study Reveals Tissue-Specific Immune Properties of Mesenchymal Stromal Cells Cédric Ménard, Joelle Dulong, David Roulois, Benjamin Hebraud, Léa Verdière, Céline Pangault, Vonick Sibut, Isabelle Bezier, Nadège Bescher, Céline Monvoisin, et al. To cite this version: Cédric Ménard, Joelle Dulong, David Roulois, Benjamin Hebraud, Léa Verdière, et al.. Inte- grated Transcriptomic, Phenotypic, and Functional Study Reveals Tissue-Specific Immune Prop- erties of Mesenchymal Stromal Cells. STEM CELLS, AlphaMed Press, 2020, 38 (1), pp.146-159. 10.1002/stem.3077. hal-02282131 HAL Id: hal-02282131 https://hal-univ-rennes1.archives-ouvertes.fr/hal-02282131 Submitted on 10 Sep 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Stem Cells Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells Journal: Stem Cells Manuscript ID Draft Wiley - Manuscript Type: Original Research Date Submitted by Forthe Peer Review n/a Author: Complete -

Islet-1 Is Essential for Pancreatic Β-Cell Function Benjamin N. Ediger
Page 1 of 68 Diabetes June 20, 2014 Diabetes Islet-1 is essential for pancreatic β-cell function Benjamin N. Ediger1,5, Aiping Du1, Jingxuan Liu1, Chad S. Hunter3, Erik R. Walp1, Jonathan Schug4, Klaus H. Kaestner4, Roland Stein3, Doris A. Stoffers5* and Catherine Lee May*,1,2 1Department of Pathology and Laboratory Medicine, Children’s Hospital of Philadelphia, 2Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA 3Department of Molecular Physiology and Biophysics, Vanderbilt University Medical Center, Nashville, Tennessee 37232, USA 4Department of Genetics and Institute for Diabetes, Obesity and Metabolism, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA 5Department of Medicine and Institute for Diabetes, Obesity and Metabolism, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA * These authors contributed equally. Running Title: Isl-1 regulates β-cell function Address correspondence to: Catherine Lee May, Ph.D. 3615 Civic Center Blvd, Room 516E Philadelphia, PA 19104 Phone: 267-426-0116 E-mail: [email protected] And Doris A. Stoffers, M.D., Ph.D. 3400 Civic Center Boulevard, 12-124 SCTR Philadelphia, PA 19104 Phone: (215) 573-5413 E-mail: [email protected] Fax: 215-590-3709 Word Count: 4065 Number of Tables: 6 (all are supplemental) Number of Figures: 9 (3 are supplemental) Diabetes Publish Ahead of Print, published online July 15, 2014 Diabetes Page 2 of 68 Abstract Isl-1 is essential for the survival and ensuing differentiation of pancreatic endocrine progenitors. Isl-1 remains expressed in all adult pancreatic endocrine lineages; however, its specific function in the postnatal pancreas is unclear. -

Identification of Genomic Targets of Krüppel-Like Factor 9 in Mouse Hippocampal
Identification of Genomic Targets of Krüppel-like Factor 9 in Mouse Hippocampal Neurons: Evidence for a role in modulating peripheral circadian clocks by Joseph R. Knoedler A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Neuroscience) in the University of Michigan 2016 Doctoral Committee: Professor Robert J. Denver, Chair Professor Daniel Goldman Professor Diane Robins Professor Audrey Seasholtz Associate Professor Bing Ye ©Joseph R. Knoedler All Rights Reserved 2016 To my parents, who never once questioned my decision to become the other kind of doctor, And to Lucy, who has pushed me to be a better person from day one. ii Acknowledgements I have a huge number of people to thank for having made it to this point, so in no particular order: -I would like to thank my adviser, Dr. Robert J. Denver, for his guidance, encouragement, and patience over the last seven years; his mentorship has been indispensable for my growth as a scientist -I would also like to thank my committee members, Drs. Audrey Seasholtz, Dan Goldman, Diane Robins and Bing Ye, for their constructive feedback and their willingness to meet in a frequently cold, windowless room across campus from where they work -I am hugely indebted to Pia Bagamasbad and Yasuhiro Kyono for teaching me almost everything I know about molecular biology and bioinformatics, and to Arasakumar Subramani for his tireless work during the home stretch to my dissertation -I am grateful for the Neuroscience Program leadership and staff, in particular -

Rabbit Anti-HIVEP3 Antibody-SL17461R
SunLong Biotech Co.,LTD Tel: 0086-571- 56623320 Fax:0086-571- 56623318 E-mail:[email protected] www.sunlongbiotech.com Rabbit Anti-HIVEP3 antibody SL17461R Product Name: HIVEP3 Chinese Name: 艾滋病病毒EP3抗体 FLJ16752; Hivep3; Human immunodeficiency virus type I enhancer-binding protein 3; Kappa-B and V(D)J recombination signal sequences-binding protein; Kappa-binding Alias: protein 1; KBP-1; KIAA1555; KRC; Schnurri-3; SHN3; Transcription factor HIVEP3; ZEP3_HUMAN; Zinc finger protein ZAS3; ZNF40C. Organism Species: Rabbit Clonality: Polyclonal React Species: Human,Mouse,Rat,Cow, ELISA=1:500-1000IHC-P=1:400-800IHC-F=1:400-800ICC=1:100-500IF=1:100- 500(Paraffin sections need antigen repair) Applications: not yet tested in other applications. optimal dilutions/concentrations should be determined by the end user. Molecular weight: 259kDa Cellular localization: The nucleuscytoplasmic Form: Lyophilized or Liquid Concentration: 1mg/ml immunogen: KLHwww.sunlongbiotech.com conjugated synthetic peptide derived from human HIVEP3:1-100/2409 Lsotype: IgG Purification: affinity purified by Protein A Storage Buffer: 0.01M TBS(pH7.4) with 1% BSA, 0.03% Proclin300 and 50% Glycerol. Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. The lyophilized antibody is stable at room temperature for at least one month and for greater than a year Storage: when kept at -20°C. When reconstituted in sterile pH 7.4 0.01M PBS or diluent of antibody the antibody is stable for at least two weeks at 2-4 °C. PubMed: PubMed This gene encodes a member of the human immunodeficiency virus type 1 enhancer- binding protein family. Members of this protein family contain multiple zinc finger and Product Detail: acid-rich (ZAS) domains and serine-threonine rich regions. -

Perkinelmer Genomics to Request the Saliva Swab Collection Kit for Patients That Cannot Provide a Blood Sample As Whole Blood Is the Preferred Sample
Autism and Intellectual Disability TRIO Panel Test Code TR002 Test Summary This test analyzes 2429 genes that have been associated with Autism and Intellectual Disability and/or disorders associated with Autism and Intellectual Disability with the analysis being performed as a TRIO Turn-Around-Time (TAT)* 3 - 5 weeks Acceptable Sample Types Whole Blood (EDTA) (Preferred sample type) DNA, Isolated Dried Blood Spots Saliva Acceptable Billing Types Self (patient) Payment Institutional Billing Commercial Insurance Indications for Testing Comprehensive test for patients with intellectual disability or global developmental delays (Moeschler et al 2014 PMID: 25157020). Comprehensive test for individuals with multiple congenital anomalies (Miller et al. 2010 PMID 20466091). Patients with autism/autism spectrum disorders (ASDs). Suspected autosomal recessive condition due to close familial relations Previously negative karyotyping and/or chromosomal microarray results. Test Description This panel analyzes 2429 genes that have been associated with Autism and ID and/or disorders associated with Autism and ID. Both sequencing and deletion/duplication (CNV) analysis will be performed on the coding regions of all genes included (unless otherwise marked). All analysis is performed utilizing Next Generation Sequencing (NGS) technology. CNV analysis is designed to detect the majority of deletions and duplications of three exons or greater in size. Smaller CNV events may also be detected and reported, but additional follow-up testing is recommended if a smaller CNV is suspected. All variants are classified according to ACMG guidelines. Condition Description Autism Spectrum Disorder (ASD) refers to a group of developmental disabilities that are typically associated with challenges of varying severity in the areas of social interaction, communication, and repetitive/restricted behaviors. -

SF3B1-Mutated Chronic Lymphocytic Leukemia Shows Evidence Of
SF3B1-mutated chronic lymphocytic leukemia shows evidence of NOTCH1 pathway activation including CD20 downregulation by Federico Pozzo, Tamara Bittolo, Erika Tissino, Filippo Vit, Elena Vendramini, Luca Laurenti, Giovanni D'Arena, Jacopo Olivieri, Gabriele Pozzato, Francesco Zaja, Annalisa Chiarenza, Francesco Di Raimondo, Antonella Zucchetto, Riccardo Bomben, Francesca Maria Rossi, Giovanni Del Poeta, Michele Dal Bo, and Valter Gattei Haematologica 2020 [Epub ahead of print] Citation: Federico Pozzo, Tamara Bittolo, Erika Tissino, Filippo Vit, Elena Vendramini, Luca Laurenti, Giovanni D'Arena, Jacopo Olivieri, Gabriele Pozzato, Francesco Zaja, Annalisa Chiarenza, Francesco Di Raimondo, Antonella Zucchetto, Riccardo Bomben, Francesca Maria Rossi, Giovanni Del Poeta, Michele Dal Bo, and Valter Gattei SF3B1-mutated chronic lymphocytic leukemia shows evidence of NOTCH1 pathway activation including CD20 downregulation. Haematologica. 2020; 105:xxx doi:10.3324/haematol.2020.261891 Publisher's Disclaimer. E-publishing ahead of print is increasingly important for the rapid dissemination of science. Haematologica is, therefore, E-publishing PDF files of an early version of manuscripts that have completed a regular peer review and have been accepted for publication. E-publishing of this PDF file has been approved by the authors. After having E-published Ahead of Print, manuscripts will then undergo technical and English editing, typesetting, proof correction and be presented for the authors' final approval; the final version of the manuscript will -

Figure S1. Gene Ontology Classification of Abeliophyllum Distichum Leaves Extract-Induced Degs
Figure S1. Gene ontology classification of Abeliophyllum distichum leaves extract-induced DEGs. The results are summarized in three main categories: Biological process, Cellular component and Molecular function. Figure S2. KEGG pathway enrichment analysis using Abeliophyllum distichum leaves extract-DEGs (A). Venn diagram analysis of DEGs involved in PI3K/Akt signaling pathway and Rap1 signaling pathway (B). Figure S3. The expression (A) and protein levels (B) of Akt3 in AL-treated SK-MEL2 cells. Values with different superscripted letters are significantly different (p < 0.05). Table S1. Abeliophyllum distichum leaves extract-induced DEGs. log2 Fold Gene name Gene description Change A2ML1 alpha-2-macroglobulin-like protein 1 isoform 2 [Homo sapiens] 3.45 A4GALT lactosylceramide 4-alpha-galactosyltransferase [Homo sapiens] −1.64 ABCB4 phosphatidylcholine translocator ABCB4 isoform A [Homo sapiens] −1.43 ABCB5 ATP-binding cassette sub-family B member 5 isoform 1 [Homo sapiens] −2.99 ABHD17C alpha/beta hydrolase domain-containing protein 17C [Homo sapiens] −1.62 ABLIM2 actin-binding LIM protein 2 isoform 1 [Homo sapiens] −2.53 ABTB2 ankyrin repeat and BTB/POZ domain-containing protein 2 [Homo sapiens] −1.48 ACACA acetyl-CoA carboxylase 1 isoform 1 [Homo sapiens] −1.76 ACACB acetyl-CoA carboxylase 2 precursor [Homo sapiens] −2.03 ACSM1 acyl-coenzyme A synthetase ACSM1, mitochondrial [Homo sapiens] −3.05 disintegrin and metalloproteinase domain-containing protein 19 preproprotein [Homo ADAM19 −1.65 sapiens] disintegrin and metalloproteinase