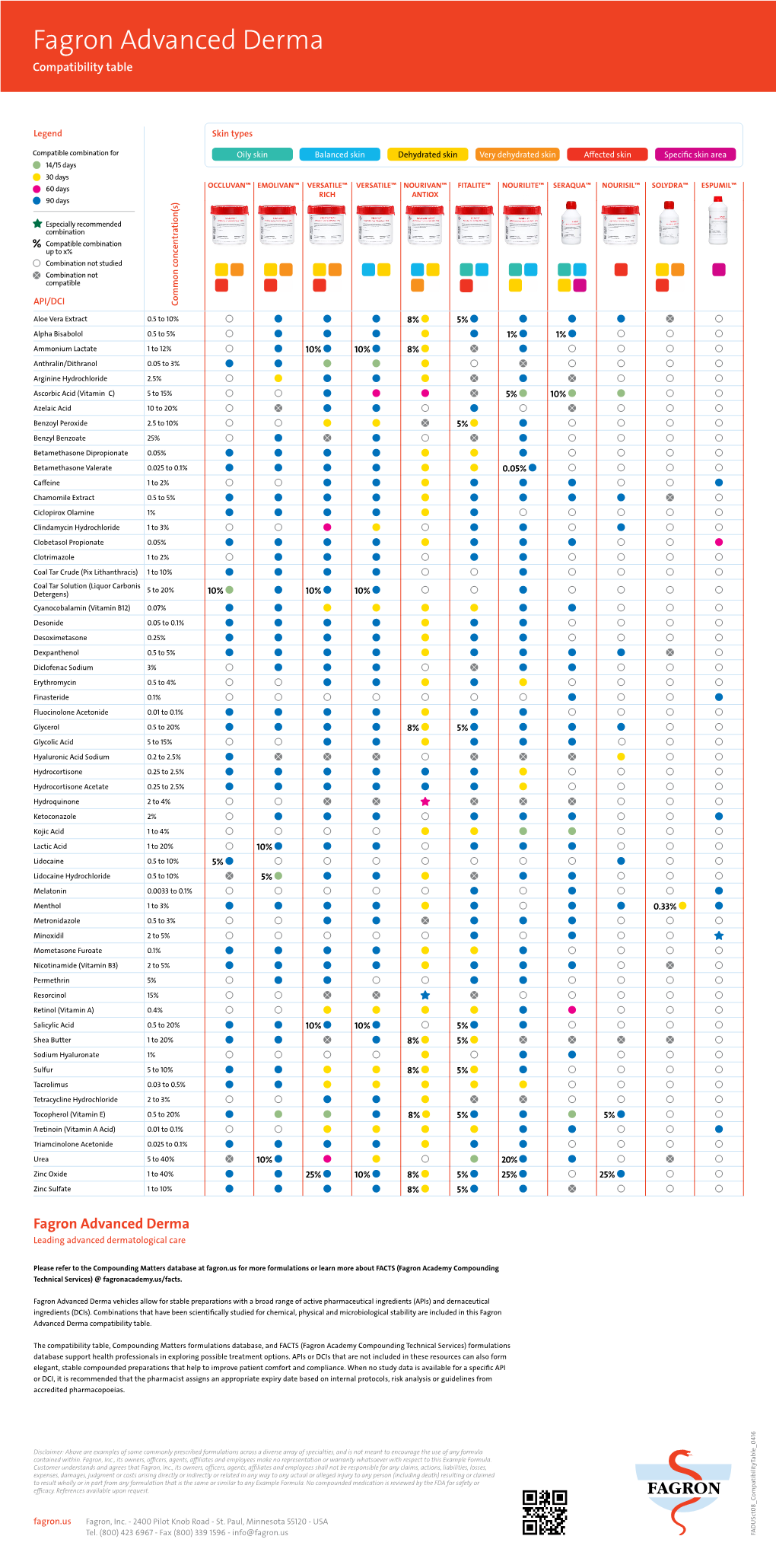
Fagron Advanced Derma Compatibility Table
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methyl Vinyl Ketone Mvk
METHYL VINYL KETONE MVK CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 30°F O.C. 20°F C.C. 7.1 Grades of Purity: 98.5+% Common Synonyms Liquid Colorless to light yellow Strong irritating 4.2 Flammable Limits in Air: 2.1% 15.6% 7.2 Storage Temperature: Cool ambient 3-Buten-2-one odor 4.3 Fire Extinguishing Agents: Dry 7.3 Inert Atmosphere: No requirement chemical, alcohol foam, carbon dioxide 7.4 Venting: Pressure-vacuum Mixes with water. Irritating vapor is produced. 4.4 Fire Extinguishing Agents Not to Be Used: Water may be ineffective. 7.5 IMO Pollution Category: Currently not available Evacuate. 4.5 Special Hazards of Combustion 7.6 Ship Type: Currently not available KEEP PEOPLE AWAY. AVOID CONTACT WITH LIQUID. Products: Not pertinent 7.7 Barge Hull Type: Currently not available Avoid inhalation. 4.6 Behavior in Fire: Vapor is heavier than Wear rubber overclothing (including gloves). air and may travel a considerable Shut off ignition sources. Call fire department. 8. HAZARD CLASSIFICATIONS distance to a source of ignition and flash Stay upwind. Use water spray to ``knock down'' vapor. back. At elevated temperatures (fire 8.1 49 CFR Category: Flammable liquid Notify local health and pollution control agencies. conditions) polymerization may take Protect water intakes. 8.2 49 CFR Class: 3 place in containers, causing violent 8.3 49 CFR Package Group: II rupture. Unburned vapors are very FLAMMABLE. irritating. 8.4 Marine Pollutant: No Fire Containers may explode in fire. 8.5 NFPA Hazard Classification: Flashback along vapor trail may occur. -

Medication List
Medication List Walgreens Plus™ members receive discounts on thousands of generic and brand-name medications included on this Medication List, which is divided into two sections, “Value Priced” Medications and “Discounted” Medications*. The price for a medication identified as “Value-Priced” is listed below: Get savings up to 85% off Cash Prices • 30-day-supply drugs cost $5 (tier 1), $10 (tier 2) or $15 (tier 3) on Atorvastatin (generic Lipitor) and • 90-day-supply drugs cost $10 (tier 1), $20 (tier 2) or $30 (tier 3) Rosuvastatin (generic Crestor) †† The Discounted Medications section lists the discounts offered to Walgreens Plus members on other generic and brand-name medications not included in the Value-Priced Medication section. The price for a medication is based on its tier and whether it is a 30-day or 90-day supply†. There may be an additional cost for quanities greater than those listed. This discount prescription pricing applies only to Walgreen Plus members on prescriptions purchased in select Walgreens stores that are not billed to insurance and/or used in combination with other health or pharmacy benefit programs. For further details, see your pharmacist or Walgreens.com/Plus. VALUE GENERICS NAPROXEN 250MG TAB 2 60 180 Antifungal NAPROXEN 500MG TAB 2 60 180 Quantity NAPROXEN 375MG TAB 2 60 180 Drug Name Tier 30 90 NAPROXEN DR 500MG TAB 3 60 180 FLUCONAZOLE 150MG TAB 2 1 3 TERBINAFINE 250MG TAB 2 30 90 Asthma Quantity Antiviral Drug Name Tier 30 90 Quantity ALBUTEROL 0.083% INH SOLN 25X3ML 2 75 225 Drug Name Tier 30 90 AMINOPHYLLINE -

Benzocaine, Lidocaine, Tetracaine)
The compounding professionals of LifeCare Pharmacy can prepare individualised therapies for a myriad of dermatological problems. Our compounding pharmacists continue to improve both the aesthetic and therapeutic aspects of customised medications, offering alternatives and advantages for dermatology. We can help you solve dermatological issues with customised formulations to meet your needs and preferences. Clear Face (Topical acne creams) Ingredients: Nicotinamide or Spironolactone topical Description: Emergence of resistant pathogens emphasizes the need for alternatives to antimicrobial agents for acne therapy. We can compound cosmetically-appealing customized formulations which can contain alternative medications to provide a synergistic effect for treatment of resistant acne. Topical nicotinamide is used in the treatment of inflammatory acne vulgaris; Topical spironolactone reduces sebum secretion rates in young adults. These alternatives to the oral tablets give the benefits of localized dispersion of the medication without the unwanted side effects of taking the oral tablets. Reference: Int J Dermatol 1995 Jun;34(6):434-7 Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne-vulgaris. Shalita AR, Smith JG, Parish LC, Sofman MS, Chalker DK Department of Dermatology, State University of New York, College of Medicine, Brooklyn, USA. J Dermatol 1996 Apr;23(4):243-6 Topical spironolactone reduces sebum secretion rates in young adults. Yamamoto A, Ito M Department of Dermatology, Niigata University School of Medicine, Japan. BLT Cream (Benzocaine, Lidocaine, Tetracaine) Ingredients: Benzocaine 20%, Lidocaine 6%, Tetracaine 4% Description: BLT Cream is commonly used to numb the area at which a patient will be undergoing laser hair removal. Particular areas that can be numbed include facial areas, and most general body areas. -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

Skin Lightening Medical Grade
MEDICAL GRADE SKIN LIGHTENING FADE-12 HAND & ARM CREAM INTENSIVE RECOVERY CREAM 1.76 oz. / 55 g. 1.7 oz. / 48 g. Advanced multi-active formula com- Contains a blend of active ingredients, Intensive Recovery Cream reduces sensi- bines 12 active ingredients including: tivity in the skin while increasing moisture skin lighteners, alpha hydroxy acids and levels and protecting against environ- botanical extracts resulting in hands mental damage. Specially formulates to which look years younger within weeks reduce irritation associates with the use of use. of alpha/beta hydroxy acids and retinoids. New EpHQuinone™ technology re- High purity extract specifcally developed sults in rapid removal of age spots for combating the irritation caused by AHA and hyperpigmentation and retinoids Contains 12 active ingredients, that Down regulates infammation enzymes to penetrates the skin and stimulates soothe the skin results Decreases Trans-Epidermal Water Loss EpHQuinone™ Interrupts the me- by 17% lanogenesis pathway at multiple points, resulting in rapid and effective Contains smart sugar that improves cell improvement in age spots and other communication for reducing stinging effect excess pigmentation. from AHA and increasing NMF (Natural Moisturizing Factor) on the skin DIRECTIONS DIRECTIONS Apply sparingly over hands and arms, Apply a generous amount to soothe allow product to absorb before applying irritated skin as needed. Allow to ab- Intensive Recovery Cream. Initial appli- sorb before applying additional prod- cation should be two to three -

Zinc Therapy in Dermatology: a Review
Hindawi Publishing Corporation Dermatology Research and Practice Volume 2014, Article ID 709152, 11 pages http://dx.doi.org/10.1155/2014/709152 Review Article Zinc Therapy in Dermatology: A Review Mrinal Gupta, Vikram K. Mahajan, Karaninder S. Mehta, and Pushpinder S. Chauhan DepartmentofDermatology,Venereology&Leprosy,Dr.R.P.Govt.MedicalCollege,Kangra(Tanda),HimachalPradesh176001,India Correspondence should be addressed to Vikram K. Mahajan; [email protected] Received 1 May 2014; Accepted 23 June 2014; Published 10 July 2014 Academic Editor: Craig G. Burkhart Copyright © 2014 Mrinal Gupta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Zinc, both in elemental or in its salt forms, has been used as a therapeutic modality for centuries. Topical preparations like zinc oxide, calamine, or zinc pyrithione have been in use as photoprotecting, soothing agents or as active ingredient of antidandruff shampoos. Its use has expanded manifold over the years for a number of dermatological conditions including infections (leishmaniasis, warts), inflammatory dermatoses (acne vulgaris, rosacea), pigmentary disorders (melasma), and neoplasias (basal cell carcinoma). Although the role of oral zinc is well-established in human zinc deficiency syndromes including acrodermatitis enteropathica, it is only in recent years that importance of zinc as a micronutrient essential for infant -

Drug Exclusions April 1, 2020
Drug Exclusions April 1, 2020 Why Doesn’t My Health Plan Cover Some Providers Drugs? To request a formulary exception for a drug noted on the list with a (+), call 877-440-0089. To request a From time to time, our pharmacy committee decides formulary exception for all other listed drugs, call the to no longer cover, or exclude, some drugs because precertification number on the member’s ID card. other safe, effective, less costly alternatives are available. The committee is composed of independent Members doctors and pharmacists who regularly review and To request a formulary exception for all drugs listed, compare prescription drugs to ensure the ones we call the Customer Service number on your ID card. cover include proven medications for treating the Note: Formulary exceptions can only be considered majority of medical conditions. with required documentation from the member’s Newly-launched products and new variations of health care provider. products already on the market are not automatically added to your health plan’s list of covered drugs, also What Happens If I Have A Prescription Filled known as a formulary. A product can be added to the For An Excluded Drug? formulary only if our pharmacy committee decides You will be able to get your drug, but you will pay the that it is clinically appropriate and cost-effective. full cost of it. What Should I Do If My Drug Is Excluded? What Drugs Are Excluded From Coverage? Talk to your doctor to see if a covered alternative drug The drugs on the following pages are excluded from is a good option for treating your condition. -

Comparison of Efficacy of Hydroquinone Versus Hydroquinone Plus Tretinoin Plus Topical Steroids in Patients with Melasma
Journal of Pakistan Association of Dermatologists. 2021;31(2):179-183. Original Article Comparison of efficacy of hydroquinone versus hydroquinone plus tretinoin plus topical steroids in patients with melasma Abeer Aslam, Leena Hafeez*, Aymon Shafi**, Asiah yousaf†, Aamina Noureen Khan**, Raheel Tahir** District Headquarter Hospital, Multan. * Khawaja Fareed Social Security Hospital, Multan. ** Nishtar Medical University and Hospital, Multan. † Tehsil Headquarter Hospital, Shujaabad. Abstract Background Melasma is an acquired pigmentary disorder characterized by symmetrical hyperpigmentation of the face. Treatment of melasma is unsatisfactory most of the times and comes with various side effects such as contact dermatitis, irritation and scarring. Objective To compare efficacy of hydroquinone versus hydroquinone plus tretinoin plus topical steroids in patients with melasma. Methods This randomized Controlled Trial was conducted in Department of Dermatology, Nishtar Medical University, Multan from 15th February 2019 to 15th August 2019. A total of 114 patients were divided in two groups, Group A, having 57 patients, was treated with hydroquinone cream once daily at bed time while group B, having 57 patients, was treated with combination therapy. Baseline and post-treatment MASI scores were calculated and patients were assessed weekly till 12 weeks and the efficacy of the treatment was documented. Efficacy was measured in terms of at least 50% reduction of MASI score after 12 weeks of therapy, compared with baseline MASI score. Results This study comprised of a total of 114 patients meeting our inclusion criteria. Of these 114, 37 (32.5%) were male patients while 77 (67.5%) were female patients. Mean age in this study was 27.22±5.08 years. -

Public Submissions on Scheduling Matters Referred to the ACMS #27, ACCS #25 and Joint ACMS-ACCS #22 Meetings Held in June 2019
Consultation: Proposed amendments to the Poisons Standard - MAY Advisory Committee on Medicines Scheduling meeting, June 2019 2019 Purpose The Pharmaceutical Society of Australia (PSA) makes this submission on proposed amendments to the Poisons Standard being referred for scheduling advice to the June 2019 meeting of the Advisory Committee on Medicines Scheduling (ACMS). PSA’s comments relate to proposed amendments to finasteride and arbutin. About PSA PSA is the only Australian Government-recognised peak national professional pharmacy organisation representing all of Australia’s 31,000 pharmacists working in all sectors and across all locations. PSA is committed to supporting pharmacists in helping Australians to access quality, safe, equitable, efficient and effective health care. PSA believes the expertise of pharmacists can be better utilised to address the health care needs of all Australians. PSA works to identify, unlock and advance opportunities for pharmacists to realise their full potential, to be appropriately recognised and fairly remunerated. PSA has a strong and engaged membership base that provides high-quality health care and are the custodians for safe and effective medicine use for the Australian community. PSA leads and supports innovative and evidence-based healthcare service delivery by pharmacists. PSA provides high-quality practitioner development and practice support to pharmacists and is the custodian of the professional practice standards and guidelines to ensure quality and integrity in the practice of pharmacy. Summary of PSA’s position Finasteride – PSA supports the proposed amendments to the Poisons Standard to include finasteride in Schedule 3 in preparations containing not more than 1 mg per dose unit in packs not greater than 30 dosage units for use in males with androgenetic alopecia. -

The Use of Compounded High Percentage Hydroquinone and Oral Tranexamic Acid in the Treatment of Resistant Melasma
The Use of Compounded High Percentage Hydroquinone and Oral Tranexamic Acid in the Treatment of Resistant Melasma ALISON TAM, D.O., F.A.O.C.D., F.A.A.D. No conflicts of interest. Which of the following are cost effective treatments for melasma? A. Compounded high percentage hydroquinones. B. Oral tranexamic Acid. C. None of the above. D. A and B. Which of the following can be used in the treatment of recalcitrant melasma? A. Tranexamic Acid 650 mg ½ tablet BID. B. Hydroquinone 12%/ Kojic Acid 6% BID. C. Hydroquinone 16%/ Kojic Acid 6% BID. D. All of the above. SUBJECTIVE Brown spots Sun Damage Acne Scars My pigment is worsening or spreading. I had a laser treatment and the brown spots are worse. I had a chemical peel and the brown spots are worse. I have been on Hydroquinone 4% for over a year and it doesn’t work. 38 year old female 60 year old female What history should you elicit now? *Your clinical dx should be done * Family History of “hyperpigmentation”? When you had treatments, were you put on hydroquinone to prep your skin before and after the treatment? What was the %? When did this start? Pregnancy, Perimenopause, OCP, Bioidentical hormones, Acne/Scarring, post peel/procedure. What else is in your skin care regimen? Vit C/antioxidants, SPF #, reapplication of SPF (unlikely), SPF in makeup (doesn’t count), tretinoin (or too irritating), aesthetician appointments. Allergies? PCN? Mushrooms? What’s your ethnicity/genetic makeup? Have you done genetic testing? What are some successful treatments that you have had in the past? You already know the diagnosis (at least in your head): Melasma PIH due to procedure Acne Scars (PIH) Combination of any of the above Plan--Melasma Although we all categorize our patients into a Fitzpatrick Skin Type, should we refine our categorization based on ethnicity? There are so many mixed races now that it becomes important to know how someone scars, develops PIH or erythema, and responds to procedures. -

List of Annexures Annexure – I Plant Layout Annexure – II Manufacturing Process Annexure – III Details of Storage of So
List of Annexures Annexure – I Plant Layout Annexure – II Manufacturing Process Annexure – III Details of Storage of Solvents Annexure – IV Water Balance & Details of Treatment and disposal of Effluent Annexure – V Details of Treatment and Disposal of Solid Waste Annexure – VI List of Raw materials & Quantity of Consumption Annexure – VII Point Source Emissions – Stack Emission Characteristics Annexure – VIII Details of Fugitive Emissions Annexure – IX Risk Identification Annexure – X Topo Map & Administrative Set-up Map Annexures 1 GCPL ANNEXURE – I PLANT LAYOUT Annexures 2 GCPL Annexures 3 GCPL Annexures 4 GCPL ANNEXURE – II MANUFACTURING PROCESS Annexures 5 GCPL Manufacturing Process Existin g Calcium Gluconate Gluconic Acid procured from market is neutralized with Calcium carbonate, filtered, crystallised, centrifuged, dried, milled and packed. NEUTRALISATION FILTERATION CRYSTALLISATION PACKING MILLING DRYING CENTRIFUGING Alternate process for the manufacture of Calcium Gluconate Gluconic acid is neutralised with Calcium carbonate, filtered, crystallised, centrifuged, dried, milled and packed. NEUTRALISATION FILTRATION CRYSTALLISATION PACKING MILLING DRYING CENTRIFUGING Alternate process GLUCONO DELTA LACTONE + CaCO + WATER 3 FILTRATION CRYSTALLISATION (NEUTRALISATION) PACKING MILLING DRYING CENTRIFUGING Annexures 6 GCPL Sodium Gluconate Gluconic acid brought from the market is neutralised with Sodium bi-carbonate, filtered, concentrated, crystallised, centrifuged, dried, milled and packed. CONCENTRATION NEUTRALISATION FILTRATION CRYSTALLISATION -

Dermatology Medication Monitoring
VA SAN DIEGO HEALTHCARE SYSTEM DERMATOLOGY DRUG FORMULARY RESTRICTED DRUGS IN ITALIC MEDICATION MONITORING IN *ASTERISK * ACNE PRODUCTS (topical) ANTI-FUNGAL (topical) SCABICIDES/PEDICULOCIDES Erythromycin 2% solution (60ml) Clotrimazole 1% cream (15g) Permethrin 1% liquid (60ml), 5% cream (60g) 1 2 Clindamycin 1% swab (#60) Ketoconazole 2% cream (30g), shampoo (120ml) Ivermectin 3mg tabs Benzoyl Peroxide 2.5%, 5%, 10% gel (60g) Metronidazole 0.75% cream (45g) EMOLLIENTS/KERATOLYTICS 5% lotion (30g) Miconazole 2% cream (30g), tincture (30ml) Ammonium lactate 12% lotion (225ml) Tretinoin 0.025%, 0.05%, 0.1% (60g) Nystatin Powder (30g) Petrolatum oint (30, 454g) Podofilox 0.5% soln (3.5ml) ACNE PRODUCTS (oral) Urea 10% lotion (240ml) Terbinafine 1% cream (30g) Doxycycline 50, 100 mg caps 10%, 20% cream (90g) Minocycline 50, 100 mg caps ANTI-PSORIATICS (topical) Salicylic acid 3% shampoo (120ml) *Isotretinoin* 10, 20, 30, 40 mg caps Coal tar Emulsion 7.5% (180ml) 40% plaster (#1) Shampoo 0.5% (255ml), 1% (180ml) Salicylic acid/Sulfur 2%/2% shampoo (120ml) ANTI-INFECTIVES (topical) Calcipotriene 0.005% cream (60g) Bacitracin 500 U (30g) TOPICAL ANESTHETICS 3 Bacitracin/polymyxin 500/10000 U/gm (30g) ANTI-PSORIATICS (oral) Capsaicin 0.025%, 0.1% cream (60g) Clindamycin phosphate 1% swab (#60) Acitretin 10, 25 mg caps Dibucaine 1% oint (30g) Mupirocin 2% oint (22g) *Azathioprine* 50 mg tabs Lidocaine 4% cream (30g), 5% oint (35g) Silver sulfadiazine 1% cream (50, 85, 400g) *Methotrexate* 2.5 mg tabs Lidocaine/Prilocaine 2.5%/2.5% cream(30g)