In Vitro Polyadenylation Is Stimulated by the Presence of an Upstream Intron
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mutation of the AAUAAA Polyadenylation Signal Depresses in Vitro Splicing of Proximal but Not Distal Introns
Downloaded from genesdev.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Mutation of the AAUAAA polyadenylation signal depresses in vitro splicing of proximal but not distal introns Maho Niwa and Susan M. Berget Marrs McClean Department of Biochemistry, Baylor College of Medicine, Houston, Texas 77030 USA To investigate the relationship between splicing and polyadenylation during the production of vertebrate mRNAs, we examined the effect of mutation of a poly(A) site on splicing of upstream introns. Mutation of the AAUAAA polyadenylation consensus sequence inhibited in vitro splicing of an upstream intron. The magnitude of the depression depended on the magnesium concentration. Dependence of splicing on polyadenylation signals suggests the existence of interaction between polyadenylation and splicing factors. In multi-intron precursor RNAs containing duplicated splice sites, mutation of the poly(A) site inhibited removal of the last intron, but not the removal of introns farther upstream. Inhibition of removal of only the last intron suggests segmental recognition of multi-exon precursor RNAs and is consistent with previous suggestions that signals at both ends of an exon are required for effective splicing of an upstream intron. [Key Words: Polyadenylation signal; splicing; vertebrate RNAs] Received July 31, 1991; revised version accepted September 10, 1991. The majority of vertebrate mRNAs undergo both splic- Using chimeric RNAs competent for both in vitro ing and polyadenylation during maturation. The rela- splicing and polyadenylation, we recently reported that tionship between the two processing steps has been un- in vitro polyadenylation is stimulated when an intron is clear. Polyadenylation has been reported to occur shortly placed upstream of a poly(A) site (Niwa et al. -

Subcellular RNA Profiling Links Splicing and Nuclear DICER1 to Alternative Cleavage and Polyadenylation
Downloaded from genome.cshlp.org on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press Subcellular RNA profiling links splicing and nuclear DICER1 to alternative cleavage and polyadenylation Jonathan Neve1#, Kaspar Burger2#, Wencheng Li3, Mainul Hoque3, Radhika Patel1, Bin Tian3, Monika Gullerova2* and Andre Furger1* Affiliations: 1 Department of Biochemistry, South Parks Road, University of Oxford, OX1 3QU, UK 2 Sir William Dunn School of Pathology, South Parks Road, University of Oxford, OX1 3RE, UK 3Department of Biochemistry and Molecular Biology, Rutgers New Jersey Medical School, Newark, NJ 07103, USA *Corresponding authors: Andre Furger Email: [email protected] Monika Gullerova Email: [email protected] # authors contributed equally Running Title: APA and subcellular fractionation Key words: alternative polyadenylation, mRNA, subcellular fractionation, DICER1 1 Downloaded from genome.cshlp.org on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press ABSTRACT Alternative cleavage and polyadenylation (APA) plays a crucial role in the regulation of gene expression across eukaryotes. Although APA is extensively studied, its regulation within cellular compartments and its physiological impact remains largely enigmatic. Here, we employed a rigorous subcellular fractionation approach to compare APA profiles of cytoplasmic and nuclear RNA fractions from human cell lines. This approach allowed us to extract APA isoforms that are subjected to differential regulation and provided us with a platform to interrogate the molecular regulatory pathways that shape APA profiles in different subcellular locations. Here we show that APA isoforms with shorter 3’UTRs tend to be overrepresented in the cytoplasm and appear to be cell type specific events. -

Coupling of Spliceosome Complexity to Intron Diversity
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.19.436190; this version posted March 20, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Coupling of spliceosome complexity to intron diversity Jade Sales-Lee1, Daniela S. Perry1, Bradley A. Bowser2, Jolene K. Diedrich3, Beiduo Rao1, Irene Beusch1, John R. Yates III3, Scott W. Roy4,6, and Hiten D. Madhani1,6,7 1Dept. of Biochemistry and Biophysics University of California – San Francisco San Francisco, CA 94158 2Dept. of Molecular and Cellular Biology University of California - Merced Merced, CA 95343 3Department of Molecular Medicine The Scripps Research Institute, La Jolla, CA 92037 4Dept. of Biology San Francisco State University San Francisco, CA 94132 5Chan-Zuckerberg Biohub San Francisco, CA 94158 6Corresponding authors: [email protected], [email protected] 7Lead Contact 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.19.436190; this version posted March 20, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. SUMMARY We determined that over 40 spliceosomal proteins are conserved between many fungal species and humans but were lost during the evolution of S. cerevisiae, an intron-poor yeast with unusually rigid splicing signals. We analyzed null mutations in a subset of these factors, most of which had not been investigated previously, in the intron-rich yeast Cryptococcus neoformans. -

Polymerse Activity
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2005 CHARACTERIZATION OF PLANT POLYADENYLATION TRANSACTING FACTORS-FACTORS THAT MODIFY POLY(A) POLYMERSE ACTIVITY Kevin Patrick Forbes University of Kentucky Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Forbes, Kevin Patrick, "CHARACTERIZATION OF PLANT POLYADENYLATION TRANSACTING FACTORS- FACTORS THAT MODIFY POLY(A) POLYMERSE ACTIVITY" (2005). University of Kentucky Doctoral Dissertations. 444. https://uknowledge.uky.edu/gradschool_diss/444 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Kevin Patrick Forbes The Graduate School University of Kentucky 2004 CHARACTERIZATION OF PLANT POLYADENYLATION TRANS- ACTING FACTORS-FACTORS THAT MODIFY POLY(A) POLYMERSE ACTIVITY _________________________________________ ABSTRACT OF DISSERTATION _________________________________________ A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Agriculture at the University of Kentucky By Kevin Patrick Forbes Lexington, Kentucky Director: Dr. Arthur G. Hunt, Professor of Agronomy Lexington, Kentucky 2004 Copyright ” Kevin Patrick Forbes 2004 ABSTRACT OF DISSERTATION CHARACTERIZATION OF PLANT POLYADENYLATION TRANS-ACTING FACTORS-FACTORS THAT MODIFY POLY(A) POLYMERSE ACTIVITY Plant polyadenylation factors have proven difficult to purify and characterize, owing to the presence of excessive nuclease activity in plant nuclear extracts, thereby precluding the identification of polyadenylation signal-dependent processing and polyadenylation in crude extracts. -

Nuclear Expression of a Group II Intron Is Consistent with Spliceosomal Intron Ancestry
Downloaded from genesdev.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Nuclear expression of a group II intron is consistent with spliceosomal intron ancestry Venkata R. Chalamcharla, M. Joan Curcio, and Marlene Belfort1 Center for Medical Sciences, Wadsworth Center, New York State Department of Health, Albany, New York 12208, USA; and School of Public Health, State University of New York at Albany, Albany, New York 12201, USA Group II introns are self-splicing RNAs found in eubacteria, archaea, and eukaryotic organelles. They are mechanistically similar to the metazoan nuclear spliceosomal introns; therefore, group II introns have been invoked as the progenitors of the eukaryotic pre-mRNA introns. However, the ability of group II introns to function outside of the bacteria-derived organelles is debatable, since they are not found in the nuclear genomes of eukaryotes. Here, we show that the Lactococcus lactis Ll.LtrB group II intron splices accurately and efficiently from different pre-mRNAs in a eukaryote, Saccharomyces cerevisiae. However, a pre-mRNA harboring a group II intron is spliced predominantly in the cytoplasm and is subject to nonsense-mediated mRNA decay (NMD), and the mature mRNA from which the group II intron is spliced is poorly translated. In contrast, a pre-mRNA bearing the Tetrahymena group I intron or the yeast spliceosomal ACT1 intron at the same location is not subject to NMD, and the mature mRNA is translated efficiently. Thus, a group II intron can splice from a nuclear transcript, but RNA instability and translation defects would have favored intron loss or evolution into protein-dependent spliceosomal introns, consistent with the bacterial group II intron ancestry hypothesis. -
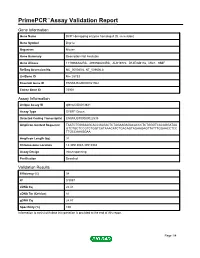
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name DCP1 decapping enzyme homolog A (S. cerevisiae) Gene Symbol Dcp1a Organism Mouse Gene Summary Description Not Available Gene Aliases 1110066A22Rik, 4930568L04Rik, AU019772, D14Ertd817e, Mitc1, SMIF RefSeq Accession No. NC_000080.6, NT_039606.8 UniGene ID Mm.28733 Ensembl Gene ID ENSMUSG00000021962 Entrez Gene ID 75901 Assay Information Unique Assay ID qMmuCID0013841 Assay Type SYBR® Green Detected Coding Transcript(s) ENSMUST00000022535 Amplicon Context Sequence TAATCTGGGAAGCACCGAGACTCTAGAAGAGACACCCTCTGGGTCACAGGATAA GTCTGCTCCGTCTGGTCATAAACATCTGACAGTAGAAGAGTTATTTGGAACCTCC TTGCCAAAGGAA Amplicon Length (bp) 91 Chromosome Location 14:30513043-30518984 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 98 R2 0.9997 cDNA Cq 22.41 cDNA Tm (Celsius) 81 gDNA Cq 24.87 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report Dcp1a, Mouse Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Mouse Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect. -

Exon Amplification: a Strategy to Isolate Mammalian Genes Based on RNA Splicing (Gene Cloning/Polymerase Chain Reaction) ALAN J
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 4005-4009, May 1991 Genetics Exon amplification: A strategy to isolate mammalian genes based on RNA splicing (gene cloning/polymerase chain reaction) ALAN J. BUCKLER*, DAVID D. CHANG, SHARON L. GRAw, J. DAVID BROOK, DANIEL A. HABER, PHILLIP A. SHARP, AND DAVID E. HOUSMAN Center for Cancer Research, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139 Contributed by Phillip A. Sharp, January 25, 1991 ABSTRACT We have developed a method, exon amplifi- (7, 8). Thus, this method may be generally applicable for the cation, for fast and efficient isolation of coding sequences from selection of exon sequences from any gene. The method is complex mammalian genomic DNA. This method is based on also both rapid and easily adapted to large scale experiments. the selection of RNA sequences, exons, which are flanked by A series of cloned genomic DNA fragments can be screened functional 5' and 3' splice sites. Fragments of cloned genomic within 1-2 weeks. The sensitivity of this method is high. DNA are inserted into an intron, which is flanked by 5' and 3' Genomic DNA segments of 20 kilobases (kb) or more can be splice sites of the human immunodeficiency virus 1 tat gene successfully screened in a single transfection by using a set contained within the plasmid pSPL1. COS-7 cells are trans- of pooled subclones. This method thus allows the rapid fected with these constructs, and the resulting RNA transcripts identification of exons in mammalian genomic DNA and are processed in vivo. Splice sites of exons contained within the should facilitate the isolation of a wide spectrum of genes of inserted genomic fragment are paired with splice sites of the significance in physiology and development. -

Mrna Turnover Philip Mitchell* and David Tollervey†
320 mRNA turnover Philip Mitchell* and David Tollervey† Nuclear RNA-binding proteins can record pre-mRNA are cotransported to the cytoplasm with the mRNP. These processing events in the structure of messenger proteins may preserve a record of the nuclear history of the ribonucleoprotein particles (mRNPs). During initial rounds of pre-mRNA in the cytoplasmic mRNP structure. This infor- translation, the mature mRNP structure is established and is mation can strongly influence the cytoplasmic fate of the monitored by mRNA surveillance systems. Competition for the mRNA and is used by mRNA surveillance systems that act cap structure links translation and subsequent mRNA as a checkpoint of mRNP integrity, particularly in the identi- degradation, which may also involve multiple deadenylases. fication of premature translation termination codons (PTCs). Addresses Cotransport of nuclear mRNA-binding proteins with mRNA Wellcome Trust Centre for Cell Biology, ICMB, University of Edinburgh, from the nucleus to the cytoplasm (nucleocytoplasmic shut- Kings’ Buildings, Edinburgh EH9 3JR, UK tling) was first observed for the heterogeneous nuclear *e-mail: [email protected] ribonucleoprotein (hnRNP) proteins. Some hnRNP proteins †e-mail: [email protected] are stripped from the mRNA at export [1], but hnRNP A1, Current Opinion in Cell Biology 2001, 13:320–325 A2, E, I and K are all exported (see [2]). Although roles for 0955-0674/01/$ — see front matter these hnRNP proteins in transport and translation have been © 2001 Elsevier Science Ltd. All rights reserved. reported [3•,4•], their affects on mRNA stability have been little studied. More is known about hnRNP D/AUF1 and Abbreviations AREs AU-rich sequence elements another nuclear RNA-binding protein, HuR, which act CBC cap-binding complex antagonistically to modulate the stability of a range of DAN deadenylating nuclease mRNAs containing AU-rich sequence elements (AREs) DSEs downstream sequence elements (reviewed in [2]). -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

The Reciprocal Regulation Between Splicing and 3-End Processing
Advanced Review The reciprocal regulation between splicing and 30-end processing Daisuke Kaida* Most eukaryotic precursor mRNAs are subjected to RNA processing events, including 50-end capping, splicing and 30-end processing. These processing events were historically studied independently; however, since the early 1990s tremendous efforts by many research groups have revealed that these processing factors interact with each other to control each other’s functions. U1 snRNP and its components negatively regulate polyadenylation of precursor mRNAs. Impor- tantly, this function is necessary for protecting the integrity of the transcriptome and for regulating gene length and the direction of transcription. In addition, physical and functional interactions occur between splicing factors and 30-end processing factors across the last exon. These interactions activate or inhibit spli- cing and 30-end processing depending on the context. Therefore, splicing and 30- end processing are reciprocally regulated in many ways through the complex protein–protein interaction network. Although interesting questions remain, future studies will illuminate the molecular mechanisms underlying the recipro- cal regulation. © 2016 The Authors. WIREs RNA published by Wiley Periodicals, Inc. How to cite this article: WIREs RNA 2016, 7:499–511. doi: 10.1002/wrna.1348 INTRODUCTION regulate each other on the transcription apparatus. In addition, because these events are carried out co- n eukaryotic cells, most precursor mRNAs (pre- transcriptionally in most cases, such factors can exist ImRNAs) consist of protein-coding regions, exons, on pre-mRNA just after synthesis of the binding sites 1,2 and intervening regions, introns. The pre-mRNAs of processing factors, suggesting that the factors are subjected to splicing to remove introns and to affect each other on the pre-mRNA. -

Sequences at the Exon-Intron Boundaries* (Split Gene/Mrna Splicing/Eukaryotic Gene Structure) R
Proc. Nati. Acad. Sci. USA Vol. 75, No. 10, pp. 4853-4857, October 1978 Biochemistry Ovalbumin gene: Evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries* (split gene/mRNA splicing/eukaryotic gene structure) R. BREATHNACH, C. BENOIST, K. O'HARE, F. GANNON, AND P. CHAMBON Laboratoire de Genetique Mol6culaire des Eucaryotes du Centre National de la Recherche Scientifique, Unite 44 de l'Institut National de la Sant6 et de la Recherche MWdicale, Institut de Chimie Biologique, Facult6 de Melecine, Strasbourg 67085, France Communicated by A. Frey-Wyssling, July 31, 1978 ABSTRACT Selected regions of cloned EcoRI fragments the 5' end of ov-mRNA and have revealed some interesting of the chicken ovalbumin gene have been sequenced. The po- features in the DNA sequences at exon-intron boundaries. sitions where the sequences coding for ovalbumin mRNA (ov- mRNA) are interrupted in the genome have been determined, and a previously unreported interruption in the DNA sequences MATERIALS AND METHODS coding for the 5' nontranslated region of the messenger has been discovered. Because directly repeated sequences are found at Plasmid pCR1 ov 2.1 containing the ov-ds-cDNA insert was exon-intron boundaries, the nucleotide sequence alone cannot prepared as described (9). EcoRI fragments "b," "c," and "d" define unique excision-ligation points for the processing of a previously cloned in X vectors (3) were transferred to the plas- possible ov-mRNA precursor. However, the sequences in these mid pBR 322. An EcoRI/HindIII of the EcoRI boundary regions share common features; this leads to the fragment proposal that there are, in fact, unique excision-ligation points fragment "a" containing the entirety of exon 7 (Fig. -

The Nuclear Poly(A) Binding Protein of Mammals, but Not of Fission Yeast, Participates in Mrna Polyadenylation
Downloaded from rnajournal.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press REPORT The nuclear poly(A) binding protein of mammals, but not of fission yeast, participates in mRNA polyadenylation UWE KÜHN, JULIANE BUSCHMANN, and ELMAR WAHLE Institute of Biochemistry and Biotechnology, Martin Luther University Halle-Wittenberg, 06099 Halle, Germany ABSTRACT The nuclear poly(A) binding protein (PABPN1) has been suggested, on the basis of biochemical evidence, to play a role in mRNA polyadenylation by strongly increasing the processivity of poly(A) polymerase. While experiments in metazoans have tended to support such a role, the results were not unequivocal, and genetic data show that the S. pombe ortholog of PABPN1, Pab2, is not involved in mRNA polyadenylation. The specific model in which PABPN1 increases the rate of poly(A) tail elongation has never been examined in vivo. Here, we have used 4-thiouridine pulse-labeling to examine the lengths of newly synthesized poly(A) tails in human cells. Knockdown of PABPN1 strongly reduced the synthesis of full-length tails of ∼250 nucleotides, as predicted from biochemical data. We have also purified S. pombe Pab2 and the S. pombe poly(A) polymerase, Pla1, and examined their in vitro activities. Whereas PABPN1 strongly increases the activity of its cognate poly(A) polymerase in vitro, Pab2 was unable to stimulate Pla1 to any significant extent. Thus, in vitro and in vivo data are consistent in supporting a role of PABPN1 but not S. pombe Pab2 in the polyadenylation of mRNA precursors. Keywords: poly(A) binding protein; poly(A) polymerase; mRNA polyadenylation; pre-mRNA 3′; processing INTRODUCTION by poly(A) polymerase with the help of the cleavage and poly- adenylation specificity factor (CPSF), which binds the polya- The poly(A) tails of eukaryotic mRNAs are covered by specif- denylation signal AAUAAA.