An Overview of Cardiac Morphogenesis. Jean-Marc Schleich, Tariq Abdulla, Ron Summers, Lucile Houyel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -
Development and Teratology of Cardiovascular and Lymphatic Systems
Development and teratology of cardiovascular and lymphatic systems Repetition: Muscle tissue Beginning of the cardiovascular system development – the 3rd week: Hemangiogenesis (day 15 – 16) – blood islets (insulae sanguinae) in extraembryonic mesoderm and splanchnic mesenchyme of embryo Clusters of mesenchyme cells (angiogenic cells) differentiate into: - angioblasts endothelium (at the periphery of blood islets) - hemoblasts primitive erythrocytes (in the center of blood islets) Clusters of angiogenic cells form a "horseshoe-shaped" space between somatic and splanchnic layer of mesoderm = pericardial cavity. Two endothelial tubes arrise in splanchnic mesoderm. The ventral portion of these tubes forms the cardiogenic area with two heart tubes, while the lateral portions form the dorsal aortae. Germ disc: prosencephalon mesencephalon eye rhombencephalon heart lateral mesoderm somites small blood vessels blood islands 8,9 Spine primitive streak Initially, the cardiogenic area is located anterior to the prechordal plate and the neural plate. The growth of the central nervous system pulls the cardiogenic area and prechordal plate (buccopharyngeal membrane ventrally and caudally ( ). Cardiogenic region just cranial to the prechordal plate. The canalization of cardiogenic clusters in the splanchnic mesoderm results in the formation of the paired heart tubes. Folding of embryo and primitive gut separation from yolk sac. Fusion of the heart tubes a single heart tube is, temporarily attached to the dorsal side of the pericardial cavity by the -

The Functional Anatomy of the Heart. Development of the Heart, Anomalies
The functional anatomy of the heart. Development of the heart, anomalies Anatomy and Clinical Anatomy Department Anastasia Bendelic Plan: Cardiovascular system – general information Heart – functional anatomy Development of the heart Abnormalities of the heart Examination in a living person Cardiovascular system Cardiovascular system (also known as vascular system, or circulatory system) consists of: 1. heart; 2. blood vessels (arteries, veins, capillaries); 3. lymphatic vessels. Blood vessels Arteries are blood vessels that carry blood away from the heart. Veins carry blood back towards the heart. Capillaries are tiny blood vessels, that connect arteries to veins. Lymphatic vessels: lymphatic capillaries; lymphatic vessels (superficial and deep lymph vessels); lymphatic trunks (jugular, subclavian, bronchomediastinal, lumbar, intestinal trunks); lymphatic ducts (thoracic duct and right lymphatic duct). Lymphatic vessels Microcirculation Microcirculatory bed comprises 7 components: 1. arterioles; 2. precapillaries or precapillary arterioles; 3. capillaries; 4. postcapillaries or postcapillary venules; 5. venules; 6. lymphatic capillaries; 7. interstitial component. Microcirculation The heart Heart is shaped as a pyramid with: an apex (directed downward, forward and to the left); a base (facing upward, backward and to the right). There are four surfaces of the heart: sternocostal (anterior) surface; diaphragmatic (inferior) surface; right pulmonary surface; left pulmonary surface. External surface of the heart The heart The heart has four chambers: right and left atria; right and left ventricles. Externally, the atria are demarcated from the ventricles by coronary sulcus (L. sulcus coronarius). The right and left ventricles are demarcated from each other by anterior and posterior interventricular sulci (L. sulci interventriculares anterior et posterior). Chambers of the heart The atria The atria are thin-walled chambers, that receive blood from the veins and pump it into the ventricles. -

The Sinus Venosus Typeof Interatrial Septal Defect*
Thorax: first published as 10.1136/thx.13.1.12 on 1 March 1958. Downloaded from Thorax (I9%8), 13, 12. THE SINUS VENOSUS TYPE OF INTERATRIAL SEPTAL DEFECT* BY H. R. S. HARLEY Cardiff (RECEIVED FOR PUBLICATION DECEMBER 30, 1957) Defects of the interatrial septum, other than namely, (1) it lies above and independent of valvular patency of the foramen ovale, are often the fossa ovalis; (2) its margin is incomplete, classified into ostium primum and ostium secun- being absent superiorly and incomplete pos- dum varieties. The relationship of the former type teriorly; and (3) it is associated with anomalous to abnormal development of the atrioventricular drainage of the right superior, and sometimes of canal has been stressed by several workers, includ- the right middle or inferior, pulmonary vein. This ing Rogers and Edwards (1948), Wakai and type of defect is illustrated in Fig. 1 (after Lewis Edwards (1956), Wakai, Swan, and Wood (1956), et al., 1955) and Fig. 2 (after Geddes, 1912). In Brandenburg and DuShane (1956), Toscano- the case reported by Ross (1956), who kindly per- Barbosa, Brandenburg, and Burchell (1956), and mitted me to see the heart, the interatrial Cooley and Kirklin (1956). These workers prefer communication was described as ". lying the term "persistent common within the orifice of atrioventricular the superior vena cava in itscopyright. canal " to "persistent ostium primum." medial wall opposite the mouths of the anomalous In addition to the above types of interatrial pulmonary veins." Ross goes on to say: "On septal defect there is a third variety, which was casual inspection of the interior of the left atrium, described as long ago as 1868 by Wagstaffe, but the defect was not visible unless a search was made which has come into prominence only since the within the superior caval orifice." The relation- http://thorax.bmj.com/ introduction of surgical repair of interatrial ship of the defect to the orifice of the superior communications under direct vision. -

Original Articles
Artigo Original %DVHV0RUIROyJLFDVSDUDR(VWXGRGR6HSWR,QWHUDWULDOQR)HWR Humano Morphological Basis for the Study of the Interatrial Septum in the Human Fetus Hugo Becker Amaral, Paulo Zielinsky, Aron Ferreira da Silveira, Ijoni Costabeber, Luiz Henrique Nicoloso, Olmiro Cezimbra de Souza Filho, Marcelo Salum, João Luiz Manica, Juliana Silveira Zanettini, Ane Micheli Costabeber 8QLGDGHGH&DUGLRORJLD)HWDOGR,QVWLWXWRGH&DUGLRORJLDGR5LR*UDQGHGR6XO'HSDUWDPHQWRGH0RUIRORJLDGR&HQWURGH&LrQFLDGD6D~GHGD Universidade Federal de Santa Maria – Porto Alegre, RS Resumo Objetivo: Descrever observações morfológicas sobre o septo interatrial em fetos normais, especialmente o forame oval e o septo primeiro, de forma a comparar a excursão do septo primeiro com o diâmetro do forame oval. Métodos: As medidas da excursão do septo primeiro (ESP) em direção ao átrio esquerdo (AE) e do diâmetro do forame oval (DFO) foram realizadas em corações de dez fetos humanos formolizados com 28 a 36 semanas. Os cortes histológicos foram feitos no FO, SP, septo segundo e nos AE e AD. Resultados: Os resultados da análise anatômica estão expressos em amplitude das medidas do DFO e da ESP: 3 fetos com idade gestacional (IG) presumida de 28 semanas, DFO (3,1-3,5 mm) e ESP (2,8-3,1 mm); 4 fetos com IG presumida de 34 semanas, DFO (3,3-3,5 mm) e ESP (4,0-5,0 mm); e 3 fetos com IG presumida de 36 semanas, DFO (3,3-4,5 mm) e ESP (6,0-9,0). Foram identificadas fibras musculares cardíacas no SP e no segundo. Conclusão: Pode-se sugerir que o SP apresenta caráter ativo devido às fibras musculares que o constituem, influenciando o fluxo sangüíneo através do FO, a mobilidade do SP e a sua excursão para o interior do AE. -
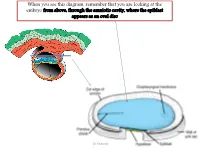
Embryo a the Development of the Heart
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc Dr. Shatarat Dr. Shatarat Why the embryo needs the vascular system? When it appears? Where it appears? with later contributions from neural crest mesenchyme Dr. Shatarat The Cardiac progenitor cells migrate from the Epiblast Through In a Cranial direction on Primitive streak each side of the notochordal process and around the prechordal plate 4 Dr. Shatarat into the splanchnic layer of the lateral plate mesoderm Dr. Shatarat into the splanchnic layer of the lateral plate mesoderm Dr. Shatarat The cells from both sides meet cranially to form the Primary Heart Field (PHF) These cells will form : • The atria • Left ventricle • Part of right ventricle • The remainder of the right ventricle • outflow tract (conus cordis and truncus arteriosus) Are derived from the Secondary Heart Field (SHF) Dr. Shatarat ONE-SOMITE AND TWO-SOMITE STAGES Dr. Shatarat Paired endothelial strands ANGIOBLASTIC CORDS appear in the cardiogenic mesoderm during the third week of development Dr. Shatarat Dr. Shatarat These cords canalize to form two heart tubes that soon fuse as embryo folds laterally to form a single heart tube late in the third week Two hearts tubs The two tubs are Fused Single heart tube is formed As the embryo folds laterally Dr. Shatarat Dr. Shatarat In addition to the cardiogenic region, other blood islands appear bilaterally, parallel and close to the midline of the embryonic shield. These islands form a pair of longitudinal vessels, the dorsal aortae. -

6. Heart and Circulatory System I
6. HEART AND CIRCULATORY SYSTEM I Dr. Taube P. Rothman P&S 12-520 [email protected] 212-305-7930 RECOMMENDED READING: Larsen Human Embryology, 3rd Edition, pp. 195-199; 157-169 top left; 172-174; bottom 181-182; 187-top 189, Simbryo-cardiovascular system SUMMARY: The circulatory system, consisting of heart, blood vessels, and blood cells is the first functional organ to develop. This lecture will focus on the formation of the embryonic vasculature, the origin and formation of the early heart tube and primitive cardiac chambers, cardiac looping, and the primitive circulation. Between the 5th - 8th week of embryonic development, the tubular heart is remodeled into a four chambered structure. We will see how right and left atrioventricular canals connect each atrium with its respective ventricle, and how the atrial septum and definitive right and left atria form. We will also see why the great veins deliver blood to the right atrium while the pulmonary veins empty into the left. GLOSSARY: Angioblasts: precursors of blood vessels Angiogenesis: lengthening, branching, sprouting and remodeling of embryonic blood vessels Aortic arches: paired arteries surrounding the pharynx; portions will contribute to formation of the great arterial vessels Blood islands: clusters of cells in the yolk sac, connecting stalk and chorionic villi that form primitive blood vessels Cardiac jelly: gelatinous extracellular matrix that forms the middle layer of the heart tube Ductus venosus: shunts most of the blood in the umbilical vein into the inferior vena cava -

Atrioventricular Node (AVN) Originates from (I) the Left Wall of Sinus Venosus and (Ii) Superior Endocardial Cushion of the Atriventricular Canal
W.S. O School of Biomedical Sciences, University of Hong Kong Objectives: • Describe early angiogenesis. • Describe the heart tube formation. • Describe the partitioning into a 4- chambered heart. • List the formation of heart valves and the conducting system in the heart. • Explain the basis of congenital heart malformation. Day 25 Day 20 Heart tubes and Intraembryonic embryonic vessels circulation established develop 6 weeks 4-5 weeks Heart tube partitions into 4 Aortic arches heart chambers transform 8 weeks Heart valves Birth completed Foramen ovale closes with increased blood flow from lungs Blood and blood vessels Primitive blood cells Angioblast (mesodermal) Endothelial cells Connective tissue surrounding mesoderm Smooth muscles Blood vessels formation The three parts of circulation: vitelline, chorionic & intraembryonic Molecular regulation of cardiac development • Heart induction – BMPs secreted by endoderm and lateral plate mesoderm together with inhibition of WNT in the anterior part of the embryo induce the expression of NKX-2.5 in the heart -forming region . • Cardiac looping - is induced by the nodal and lefty2 genes. These two genes induce the transcription factor of PITX2 in deposition of extracellular matrix in looping. Development of the heart - 1 1. Cardiogenic plate appears around 3rd week Anterior to prochordal plate Pericardial coelom develop around heart tube 2. Rotation of heart primordia –180 rotation 3. Fusion of heart tubes around 4th week; 4. Differentiation of the heart tube tissue Epicardium – connective tissue Myocardium – cardiac muscle Subendocardium – spongy reticular tissue Endocardium - endothelium Embryonic development of the heart. (a) Endocardial heart tubes start to fuse, (b) Fusion of heart tubes complete, (c) Division of heart tube into dilated segments, (d) Looping of the heart tube, (e) Looping complete, (f) Frontal section of heart tube. -

Development of CVS I
Development of CVS I Systemic Embryology Heart development (cardiogenesis) • Prenatal development of the human heart • Begins with the formation of two endocardial tubes • These tubes merge to form the tubular heart, also called the primitive heart tube • Primitive heart tube loops and septates into four chambers and paired arterial trunks that form the adult heart. 2 3 Early development of the heart 4 • First major system to function in the embryo. • Primordial heart and vascular system appear in the middle of the third week (Day 21or 22). - The rapidly growing embryo can no longer satisfy its nutritional and oxygen requirements by diffusion alone. - A need for an efficient method of acquiring oxygen and nutrients from the maternal blood and disposing of carbon dioxide and waste products. 5 Derived from: • Splanchnic mesoderm, which forms the primordium of the heart • Paraxial and lateral mesoderm • Pharyngeal mesoderm • Neural crest cells 6 • In the splanchnopleuric mesenchyme on either side of the neural plate, a horseshoe-shaped area develops as the cardiogenic region • Formed from cardiac myoblasts and blood islands • By day 19, an endocardial tube begins to develop in each side of this region • Tubes grow, converge and merge to form a single tube – the tubular heart by programmed cell death 7 • Cardiac progenitor cells in the epiblast, lateral to the primitive streak migrate through the streak. • Cardiogenic region develops cranially and laterally to the neural plate • As embryonic folding continues, the two endocardial tubes are pushed into the thoracic cavity, where they fuse together at about the 22nd day 8 • Heart develop near the head of the embryo in the cardiogenic region • Cells in the splanchnic layer of the lateral plate mesoderm are induced by pharyngeal endoderm to form cardiac myoblasts. -

The Sinus Venosus Typeof Interatrial Septal Defect*
Thorax (I9%8), 13, 12. THE SINUS VENOSUS TYPE OF INTERATRIAL SEPTAL DEFECT* BY H. R. S. HARLEY Cardiff (RECEIVED FOR PUBLICATION DECEMBER 30, 1957) Defects of the interatrial septum, other than namely, (1) it lies above and independent of valvular patency of the foramen ovale, are often the fossa ovalis; (2) its margin is incomplete, classified into ostium primum and ostium secun- being absent superiorly and incomplete pos- dum varieties. The relationship of the former type teriorly; and (3) it is associated with anomalous to abnormal development of the atrioventricular drainage of the right superior, and sometimes of canal has been stressed by several workers, includ- the right middle or inferior, pulmonary vein. This ing Rogers and Edwards (1948), Wakai and type of defect is illustrated in Fig. 1 (after Lewis Edwards (1956), Wakai, Swan, and Wood (1956), et al., 1955) and Fig. 2 (after Geddes, 1912). In Brandenburg and DuShane (1956), Toscano- the case reported by Ross (1956), who kindly per- Barbosa, Brandenburg, and Burchell (1956), and mitted me to see the heart, the interatrial Cooley and Kirklin (1956). These workers prefer communication was described as ". lying the term "persistent common atrioventricular within the orifice of the superior vena cava in its canal " to "persistent ostium primum." medial wall opposite the mouths of the anomalous In addition to the above types of interatrial pulmonary veins." Ross goes on to say: "On septal defect there is a third variety, which was casual inspection of the interior of the left atrium, described as long ago as 1868 by Wagstaffe, but the defect was not visible unless a search was made which has come into prominence only since the within the superior caval orifice." The relation- introduction of surgical repair of interatrial ship of the defect to the orifice of the superior communications under direct vision. -

Closure Devices for Patent Foramen Ovale and Atrial Septal Defects
MEDICAL POLICY – 2.02.09 Closure Devices for Patent Foramen Ovale and Atrial Septal Defects BCBSA Ref. Policy: 2.02.09 Effective Date: Aug. 1, 2021 RELATED MEDICAL POLICIES: Last Revised: July 9, 2021 None Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction The heart’s two upper chambers are called atria. The septum is the thin wall of tissue that separates the atria. Sometimes there may be a congenital heart defect in which there is a hole in the septum between the two atria. This is called an atrial septal defect. Atrial septal defects may not cause any problems and might not even be diagnosed until adulthood. Larger atrial septal defects may cause problems and may need to be closed. One way to treat an atrial septal defect is to use a catheter. A catheter is a long, thin tube which is threaded through a blood vessel in the groin to the heart. Once the catheter is in the correct location, a small device is put in place to seal the opening between the atria. This policy discusses when a catheter may be considered medically necessary to treat atrial septal defects. The foramen ovale is an opening in the septum between the two atria that is normally found in a baby before it is born. This opening usually closes soon after birth. If a foramen ovale doesn’t automatically close after the baby is born, it is called a patent foramen ovale (PFO). -

Development and Teratology of Cardiovascular and Lymphatic Systems
Development and teratology of cardiovascular and lymphatic systems in the 3rd week: hemangiogenesis (day 15 – 16) blood islets (insulae sanguinae) in extraembryonic mesoderm and splanchnic mesenchyme of embryo Differentiation of mesenchymal cells angiogenic cells: - angioblasts endothelium (at the periphery of blood islets) - hemoblasts primitive erythrocytes (in the center of blood islets) Clusters of angiogenic cells form a "horseshoe-shaped" space between somatic and splanchnic layer of mesoderm = pericardial cavity. Two endothelial tubes arrise in splanchnic mesoderm. The ventral portion of these tubes forms the cardiogenic area with two heart tubes, while the lateral portions form the dorsal aortae. Initially, the cardiogenic area is located anterior to the prechordal plate and the neural plate. The growth of the central nervous system pulls the cardiogenic area and prechordal plate (buccopharyngeal membrane ventrally and caudally ( ). Cardiogenic region just cranial to the prechordal plate. The canalization of cardiogenic clusters in the splanchnic mesoderm results in the formation of the paired heart tubes. Folding of embryo and primitive gut separation from yolk sac. Fusion of the heart tubes a single heart tube is, temporarily attached to the dorsal side of the pericardial cavity by the dorsal mesocardium (4). The splanchnic mesoderm (5) around the heart tube (3 - future endocardium) thickens and forms the heart jelly = myoepicardial mantle (future myocardium and epicardium). 3 Heart tube Cor Truncus arteriosus tubulare + aortic roots Bulbus cordis Cor Ventricle sigmoideum Atrium Sinus venosus Cor Common quadricameratum cardinal veins Umbilical veins Vitelline veins Looping of the heart tube - because growth of the heart tube is faster then growth of the pericardial cavity Cor quadricameratum Atrioventricular septum Endocardial cushions develop in the dorsal and ventral walls of the heart in the region of the atrioventricular canal.