Androstenedione, Dehydroepiandrosterone and Testosterone in Ovarian Vein Plasma and Androstenedione in Peripheral Arterial Plasma During the Bovine Oestrous Cycle T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dehydroepiandrosterone – Is the Fountain of Youth Drying Out?
Physiol. Res. 52: 397-407, 2003 MINIREVIEW Dehydroepiandrosterone – Is the Fountain of Youth Drying Out? P. CELEC 1,2, L. STÁRKA3 1Faculty of Medicine, 2Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia and 3Institute of Endocrinology, Prague, Czech Republic Received September 15, 2002 Accepted October 7, 2002 Summary Dehydroepiandrosterone (DHEA) and its sulphate-bound form (DHEAS) are important steroids mainly of adrenal origin. Their physiological and pathophysiological functions are not yet fully identified, although a number of various possible features have been hypothesized. Most popular is the description of the “hormone of youth” as the long-term dynamics of DHEA levels are characterized by a sharp age-related decline in the late adulthood and later. Low levels of DHEA are, however, associated not only with the ageing process but also with diabetes mellitus, cardiovascular diseases and some neurological or immunological entities. In the past decade, a number of brief studies have concentrated on these relationships and also on the role of exogenous DHEA in health, disease and human well-being. This article tries to summarize some of the most important facts achieved recently. Key words Dehydroepiandrosterone • Intracrinology • Hormone replacement therapy • Steroids Introduction functions: 1) DHEA is an endogenous metabolite that cannot be patented so that pharmaceutical companies are In 1934 Butenandt and Dannenbaum isolated not interested in supporting research in this field. dehydroepiandrosterone (DHEA) from urine and in 1944 2) DHEA can be described as a “human molecule” Munson and colleagues identified its 3β-sulphate because other investigated species have much lower (DHEAS). Even now, nearly 70 years later, we still do concentrations. -

Reproductive DHEA-S
Reproductive DHEA-S Analyte Information - 1 - DHEA-S Introduction DHEA-S, DHEA sulfate or dehydroepiandrosterone sulfate, it is a metabolite of dehydroepiandrosterone (DHEA) resulting from the addition of a sulfate group. It is the sulfate form of aromatic C19 steroid with 10,13-dimethyl, 3-hydroxy group and 17-ketone. Its chemical name is 3β-hydroxy-5-androsten-17-one sulfate, its summary formula is C19H28O5S and its molecular weight (Mr) is 368.5 Da. The structural formula of DHEA-S is shown in (Fig.1). Fig.1: Structural formula of DHEA-S Other names used for DHEA-S include: Dehydroisoandrosterone sulfate, (3beta)-3- (sulfooxy), androst-5-en-17-one, 3beta-hydroxy-androst-5-en-17-one hydrogen sulfate, Prasterone sulfate and so on. As DHEA-S is very closely connected with DHEA, both hormones are mentioned together in the following text. Biosynthesis DHEA-S is the major C19 steroid and is a precursor in testosterone and estrogen biosynthesis. DHEA-S originates almost exclusively in the zona reticularis of the adrenal cortex (Fig.2). Some may be produced by the testes, none is produced by the ovaries. The adrenal gland is the sole source of this steroid in women, whereas in men the testes secrete 5% of DHEA-S and 10 – 20% of DHEA. The production of DHEA-S and DHEA is regulated by adrenocorticotropin (ACTH). Corticotropin-releasing hormone (CRH) and, to a lesser extent, arginine vasopressin (AVP) stimulate the release of adrenocorticotropin (ACTH) from the anterior pituitary gland (Fig.3). In turn, ACTH stimulates the adrenal cortex to secrete DHEA and DHEA-S, in addition to cortisol. -

Safety Data Sheet
SAFETY DATA SHEET SECTION 1: PRODUCT IDENTIFICATION PRODUCT NAME DHEA (Prasterone) (Micronized) PRODUCT CODE 0733 SUPPLIER MEDISCA Inc. Tel.: 1.800.932.1039 | Fax.: 1.855.850.5855 661 Route 3, Unit C, Plattsburgh, NY, 12901 3955 W. Mesa Vista Ave., Unit A-10, Las Vegas, NV, 89118 6641 N. Belt Line Road, Suite 130, Irving, TX, 75063 MEDISCA Pharmaceutique Inc. Tel.: 1.800.665.6334 | Fax.: 514.338.1693 4509 Rue Dobrin, St. Laurent, QC, H4R 2L8 21300 Gordon Way, Unit 153/158, Richmond, BC V6W 1M2 MEDISCA Australia PTY LTD Tel.: 1.300.786.392 | Fax.: 61.2.9700.9047 Unit 7, Heritage Business Park 5-9 Ricketty Street, Mascot, NSW 2020 EMERGENCY PHONE CHEMTREC Day or Night Within USA and Canada: 1-800-424-9300 NSW Poisons Information Centre: 131 126 USES Adjuvant; Androgen SECTION 2: HAZARDS IDENTIFICATION GHS CLASSIFICATION Toxic to Reproduction (Category 2) PICTOGRAM SIGNAL WORD Warning HAZARD STATEMENT(S) Reproductive effector, prohormone. Suspected of damaging fertility or the unborn child. May cause harm to breast-fed children. Causes serious eye irritation. Causes skin and respiratory irritation. Very toxic to aquatic life with long lasting effects. AUSTRALIA-ONLY HAZARDS Not Applicable. PRECAUTIONARY STATEMENT(S) Prevention Wash thoroughly after handling. Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Do not breathe dusts or mists. Do not eat, drink or smoke when using this product. Avoid contact during pregnancy/while nursing. Wear protective gloves, protective clothing, eye protection, face protection. Avoid release to the environment. Response IF ON SKIN (HAIR): Wash with plenty of water. -

A10 Anabolic Steroids Hardcore Info
CONTENTS GENERAL INFORMATION 3 Anabolic steroids – What are they? 4 How do they Work? – Aromatisation 5 More molecules – More problems 6 The side effects of anabolic steroids 7 Women and anabolic steroids 8 Injecting steroids 9 Abscesses – Needle Exchanges 10 Intramuscular injection 11 Injection sites 12 Oral steroids – Cycles – Stacking 13 Diet 14 Where do steroids come from? Spotting a counterfeit 15 Drug Information – Drug dosage STEROIDS 16 Anadrol – Andriol 17 Anavar – Deca-Durabolin 18 Dynabolon – Durabolin – Dianabol 19 Esiclene – Equipoise 20 Primobolan Depot – Proviron – Primobolan orals – Pronobol 21 Sustanon – Stromba, Strombaject – Testosterone Cypionate Testosterone Enanthate 22 Testosterone Propionate – Testosterone Suspension 23 Trenbolone Acetate – Winstrol OTHER DRUGS 24 Aldactone – Arimidex 25 Clenbuterol – Cytomel 26 Ephedrine Hydrochloride – GHB 27 Growth Hormone 28 Insulin 30 Insulin-Like Growth Factor-1 – Human Chorionic Gonadotrophin 31 Tamoxifen – Nubain – Recreational Drugs 32 Steroids and the Law 34 Glossary ANABOLIC STEROIDS People use anabolic steroids for various reasons, some use them to build muscle for their job, others just want to look good and some use them to help them in sport or body building. Whatever the reason, care needs to be taken so that as little harm is done to the body as possible because despite having muscle building effects they also have serious side effects especially when used incorrectly. WHAT ARE THEY? Anabolic steroids are man made versions of the hormone testosterone. Testosterone is the chemical in men responsible for facial hair, deepening of the voice and sex organ development, basically the masculine things Steroids are in a man. used in medicine to treat anaemia, muscle weakness after These masculine effects surgery etc, vascular are called the androgenic disorders and effects of testosterone. -

Pharmacology/Therapeutics II Block III Lectures 2013-14
Pharmacology/Therapeutics II Block III Lectures 2013‐14 66. Hypothalamic/pituitary Hormones ‐ Rana 67. Estrogens and Progesterone I ‐ Rana 68. Estrogens and Progesterone II ‐ Rana 69. Androgens ‐ Rana 70. Thyroid/Anti‐Thyroid Drugs – Patel 71. Calcium Metabolism – Patel 72. Adrenocorticosterioids and Antagonists – Clipstone 73. Diabetes Drugs I – Clipstone 74. Diabetes Drugs II ‐ Clipstone Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones Date: Thursday, March 20, 2014-8:30 AM Reading Assignment: Katzung, Chapter 37 Key Concepts and Learning Objectives To review the physiology of neuroendocrine regulation To discuss the use neuroendocrine agents for the treatment of representative neuroendocrine disorders: growth hormone deficiency/excess, infertility, hyperprolactinemia Drugs discussed Growth Hormone Deficiency: . Recombinant hGH . Synthetic GHRH, Recombinant IGF-1 Growth Hormone Excess: . Somatostatin analogue . GH receptor antagonist . Dopamine receptor agonist Infertility and other endocrine related disorders: . Human menopausal and recombinant gonadotropins . GnRH agonists as activators . GnRH agonists as inhibitors . GnRH receptor antagonists Hyperprolactinemia: . Dopamine receptor agonists 1 Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. 1. Overview of Neuroendocrine Systems The neuroendocrine -

The Role of Highly Selective Androgen Receptor (AR) Targeted
P h a s e I I S t u d y o f I t r a c o n a z o l e i n B i o c h e m i c a l R e l a p s e Version 4.0: October 8, 2014 CC# 125513 CC# 125513: Hedgehog Inhibition as a Non-Castrating Approach to Hormone Sensitive Prostate Cancer: A Phase II Study of Itraconazole in Biochemical Relapse Investigational Agent: Itraconazole IND: IND Exempt (IND 116597) Protocol Version: 4.0 Version Date: October 8, 2014 Principal Investigator: Rahul Aggarwal, M.D., HS Assistant Clinical Professor Division of Hematology/Oncology, Department of Medicine University of California San Francisco 1600 Divisadero St. San Francisco, CA94115 [email protected] UCSF Co-Investigators: Charles J. Ryan, M.D., Eric Small, M.D., Professor of Medicine Professor of Medicine and Urology Lawrence Fong, M.D., Terence Friedlander, M.D., Professor in Residence Assistant Clinical Professor Amy Lin, M.D., Associate Clinical Professor Won Kim, M.D., Assistant Clinical Professor Statistician: Li Zhang, Ph.D, Biostatistics Core RevisionHistory October 8, 2014 Version 4.0 November 18, 2013 Version 3.0 January 28, 2013 Version 2.0 July 16, 2012 Version 1.0 Phase II - Itraconazole Page 1 of 79 P h a s e I I S t u d y o f I t r a c o n a z o l e i n B i o c h e m i c a l R e l a p s e Version 4.0: October 8, 2014 CC# 125513 Protocol Signature Page Protocol No.: 122513 Version # and Date: 4.0 - October 8, 2014 1. -

Hyperandrogenic States in Women: Pitfalls in Laboratory Diagnosis
4 178 M Pugeat and others Laboratory diagnosis of 178:4 R141–R154 Review hyperandrogenic states MANAGEMENT OF ENDOCRINE DISEASE Hyperandrogenic states in women: pitfalls in laboratory diagnosis Michel Pugeat1,2,3, Ingrid Plotton2,4, Aude Brac de la Perrière1, Gérald Raverot1,2, Henri Déchaud1,2 and Véronique Raverot4 1Fédération d’Endocrinologie, Groupement Hospitalier Est, Hospices Civils de Lyon, Bron, France, 2Université Claude Correspondence Bernard Lyon 1, Lyon, France, 3INSERM U1060 Institut CarMen, Lyon, France, and 4Laboratoire d’Hormonologie, should be addressed d’Endocrinologie Moléculaire et des Maladies Rares, Groupement Hospitalier Est, Hospices Civils de Lyon, to M Pugeat Bron, France Email [email protected] Abstract Measuring total testosterone level is the first-line approach in assessing androgen excess in women. The main pitfalls in measuring testosterone relate to its low concentration and to the structural similarity between circulating androgens and testosterone, requiring accurate techniques with high specificity and sensitivity. These goals can be achieved by immunoassay using a specific anti-testosterone monoclonal antibody, ideally after an extraction step. Liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) will be commonly used for measuring testosterone, providing optimal accuracy with a low limit of detection. Yet, the pitfalls of these two techniques are well identified and must be recognized and systematically addressed. In general, laboratories using direct testosterone immunoassay and mass spectrometry need to operate within a quality framework and be actively engaged in external quality control processes and standardization, so as to ensure appropriate interpretation irrespective of the particular laboratory. Circulating testosterone is strongly bound to sex-hormone-binding globulin (SHBG), and SHBG levels are typically low in overweight hyperandrogenic patients. -

Androstenedione, LC/MS/MS Androstenedione, •
Androstenedione, LC/MS/MS 17182X Clinical Use Clinical Background • Diagnose hirsutism, polycystic Androstenedione is an androgenic ovarian disease, virilization, and steroid hormone produced by both the congenital adrenal hyperplasia adrenal cortex and the gonads. In males, the compound's activity is Test Alphabetical Reference Range relatively unimportant, compared with Section ng/dL the predominant testicular androgen Men testosterone. In females, andros- 18-30 y 50-220 tenedione–and the testosterone and 31-50 y 40-190 dihydrotestosterone to which it is 51-60 y 50-220 metabolized–normally contribute to Women the growth of sexually dependent Follicular 35-250 axillary and pubic hair. In women, the Midcycle 60-285 concentration of androstenedione Luteal 30-235 varies with the menstrual cycle, with Postmenopausal 20-75 the ovaries and adrenals contributing equally during the follicular phase. At Children mid-cycle, increased ovarian andros- 1-12 m 6-78 tenedione secretion accounts for 70% 1-4 y 5-51 of its overall production. 5-9 y 6-115 10-13 y 12-221 Excessive androstenedione production 14-17 y 22-225 may cause hirsutism and contribute to Tanner II-III virilization. This can occur in the polycystic ovary syndrome, idiopathic Males 17-82 hirsutism, ovarian and adrenal Females 43-180 neoplasms, and congenital adrenal Tanner IV-V hyperplasia due to 21-hydroxylase or Males 57-150 17β-hydroxylase deficiencies. In Females 7-68 hirsute patients with an elevated Pediatric data from J Clin Endocrinol Metab. androstenedione concentration, serial 1991;73:674-686 and J Clin Endocrinol Metab. measurements are useful to monitor 1989;69:1133-1136. -

Combined Oral Contraceptives Plus Spironolactone Compared With
177:5 M Alpañés, F Álvarez-Blasco Randomized trial of common 177:5 399–408 Clinical Study and others drugs for PCOS Combined oral contraceptives plus spironolactone compared with metformin in women with polycystic ovary syndrome: a one-year randomized clinical trial Macarena Alpañés*, Francisco Álvarez-Blasco*, Elena Fernández-Durán, Manuel Luque-Ramírez and Héctor F Escobar-Morreale Correspondence Diabetes, Obesity and Human Reproduction Research Group, Department of Endocrinology & Nutrition, Hospital should be addressed Universitario Ramón y Cajal & Universidad de Alcalá & Instituto Ramón y Cajal de Investigación Sanitaria IRYCIS & to H F Escobar-Morreale Centro de Investigación Biomédica en Red Diabetes y Enfermedades Metabólicas Asociadas CIBERDEM, Madrid, Spain Email *(M Alpañés and F Álvarez-Blasco contributed equally to this work) hectorfrancisco.escobar@ salud.madrid.org Abstract Objective: We aimed to compare a combined oral contraceptive (COC) plus the antiandrogen spironolactone with the insulin sensitizer metformin in women with polycystic ovary syndrome (PCOS). Design: We conducted a randomized, parallel, open-label, clinical trial comparing COC (30 μg of ethinylestradiol and 150 μg of desogestrel) plus spironolactone (100 mg/day) with metformin (850 mg b.i.d.) for one year in women with PCOS (EudraCT2008–004531–38). Methods: The composite primary outcome included efficacy (amelioration of hirsutism, androgen excess and menstrual dysfunction) and cardiometabolic safety (changes in the frequencies of disorders of glucose tolerance, dyslipidemia and hypertension). A complete anthropometric, biochemical, hormonal and metabolic evaluation was conducted every three months and data were submitted to intention-to-treat analyses. European Journal European of Endocrinology Results: Twenty-four patients were assigned to COC plus spironolactone and 22 patients to metformin. -

Centene Employee
Centene Employee PREFERRED DRUG LIST The Centene Employee Formulary includes a list of drugs covered by your prescription benefit. The formulary is updated often and may change. To get the most up-to-date information, you may view the latest formulary on our website at https://pharmacy.envolvehealth.com/members/formulary.html or call us at 1-844-262-6337. Updated: December 1, 2020 Table of Contents What is the Centene Employee Formulary?...................... .....................................................................ii How are the drugs listed in the categorical list?................................... .................................................ii How much will I pay for my drugs?.......................................................................................................ii How do I find a drug on the Drug List?..................................................................................................iii Are there any limits on my drug coverage?.............................................................................................iv Can I go to any pharmacy?......................................................................................................................iv Can I use a mail order pharmacy?...........................................................................................................v How can I get prior authorization or an exception to the rules for drug coverage?................................v How can I save money on my prescription drugs?.................................................................................v -

Pros and Cons Controversy on Molecular Imaging and Dynamic
Open Access Archives of Biotechnology and Biomedicine Research Article Pros and Cons Controversy on Molecular Imaging and Dynamics of Double- ISSN Standard DNA/RNA of Human Preserving 2639-6777 Stem Cells-Binding Nano Molecules with Androgens/Anabolic Steroids (AAS) or Testosterone Derivatives through Tracking of Helium-4 Nucleus (Alpha Particle) Using Synchrotron Radiation Alireza Heidari* Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA *Address for Correspondence: Dr. Alireza Abstract Heidari, Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, In the current study, we have investigated pros and cons controversy on molecular imaging and dynamics USA, Email: of double-standard DNA/RNA of human preserving stem cells-binding Nano molecules with Androgens/ [email protected]; Anabolic Steroids (AAS) or Testosterone derivatives through tracking of Helium-4 nucleus (Alpha particle) using [email protected] synchrotron radiation. In this regard, the enzymatic oxidation of double-standard DNA/RNA of human preserving Submitted: 31 October 2017 stem cells-binding Nano molecules by haem peroxidases (or heme peroxidases) such as Horseradish Peroxidase Approved: 13 November 2017 (HPR), Chloroperoxidase (CPO), Lactoperoxidase (LPO) and Lignin Peroxidase (LiP) is an important process from Published: 15 November 2017 both the synthetic and mechanistic point of view. Copyright: 2017 Heidari A. This is an open access article distributed under the Creative -
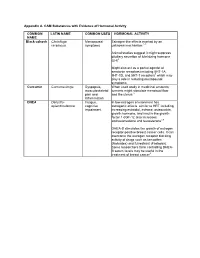
Appendix A. CAM Substances with Evidence of Hormonal Activity
Appendix A. CAM Substances with Evidence of Hormonal Activity COMMON LATIN NAME COMMON USES HORMONAL ACTIVITY NAME Black cohosh Cimicifuga Menopausal Estrogen-like effects exerted by an racemosa symptoms unknown mechanism 1-3 Animal studies suggest it might suppress pituitary secretion of luteinizing hormone (LH) 4 Might also act as a partial agonist at serotonin receptors including 5HT-1A, 5HT-1D, and 5HT-7 receptors 5 which may play a role in reducing menopausal symptoms Curcumin Curcuma longa Dyspepsia, When used orally in medicinal amounts; musculoskeletal turmeric might stimulate menstrual flow pain and and the uterus 6 inflammation DHEA Dehydro- Fatigue, In low-estrogen environment has epiandrosterone cognitive estrogenic effects similar to HRT including impairment increasing estradiol, estrone, osteocalcin, growth hormone, and insulin-like growth factor 1 (IGF-1); also increases androstenedione and testosterone 7,8 DHEA-S stimulates the growth of estrogen receptor-positive breast cancer cells. It can overcome the estrogen receptor blocking activity of drugs such as tamoxifen (Nolvadex) and fulvestrant (Faslodex). Some researchers think controlling DHEA- S serum levels may be useful in the treatment of breast cancer 9 Dong quai Angelica sinensis Dysmenorrhea, Research suggests estrogenic effects 10 , 11 menopausal 12 13 symptoms Competitively inhibits estradiol binding to estrogen receptors and induces transcription activity in estrogen- responsive cells 10 Ferulic acid stimulates proliferation of estrogen-receptor positive breast cancer cells in vitro. It also appears to up-regulate transcription of HER2 oncogene and ESR1 gene 14 Stimulates proliferation of both estrogen- receptor positive and negative breast cancer cells in vitro. The effect on estrogen-receptor positive cells appears to involve estrogen agonist activity.