1 Cryo-EM Structures of Respiratory Bc1-Cbb3 Type CIII2CIV
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
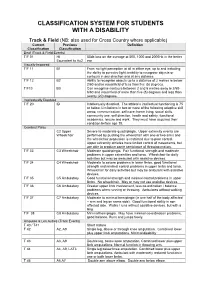
Disability Classification System
CLASSIFICATION SYSTEM FOR STUDENTS WITH A DISABILITY Track & Field (NB: also used for Cross Country where applicable) Current Previous Definition Classification Classification Deaf (Track & Field Events) T/F 01 HI 55db loss on the average at 500, 1000 and 2000Hz in the better Equivalent to Au2 ear Visually Impaired T/F 11 B1 From no light perception at all in either eye, up to and including the ability to perceive light; inability to recognise objects or contours in any direction and at any distance. T/F 12 B2 Ability to recognise objects up to a distance of 2 metres ie below 2/60 and/or visual field of less than five (5) degrees. T/F13 B3 Can recognise contours between 2 and 6 metres away ie 2/60- 6/60 and visual field of more than five (5) degrees and less than twenty (20) degrees. Intellectually Disabled T/F 20 ID Intellectually disabled. The athlete’s intellectual functioning is 75 or below. Limitations in two or more of the following adaptive skill areas; communication, self-care; home living, social skills, community use, self direction, health and safety, functional academics, leisure and work. They must have acquired their condition before age 18. Cerebral Palsy C2 Upper Severe to moderate quadriplegia. Upper extremity events are Wheelchair performed by pushing the wheelchair with one or two arms and the wheelchair propulsion is restricted due to poor control. Upper extremity athletes have limited control of movements, but are able to produce some semblance of throwing motion. T/F 33 C3 Wheelchair Moderate quadriplegia. Fair functional strength and moderate problems in upper extremities and torso. -

MIPS IV Instruction Set
MIPS IV Instruction Set Revision 3.2 September, 1995 Charles Price MIPS Technologies, Inc. All Right Reserved RESTRICTED RIGHTS LEGEND Use, duplication, or disclosure of the technical data contained in this document by the Government is subject to restrictions as set forth in subdivision (c) (1) (ii) of the Rights in Technical Data and Computer Software clause at DFARS 52.227-7013 and / or in similar or successor clauses in the FAR, or in the DOD or NASA FAR Supplement. Unpublished rights reserved under the Copyright Laws of the United States. Contractor / manufacturer is MIPS Technologies, Inc., 2011 N. Shoreline Blvd., Mountain View, CA 94039-7311. R2000, R3000, R6000, R4000, R4400, R4200, R8000, R4300 and R10000 are trademarks of MIPS Technologies, Inc. MIPS and R3000 are registered trademarks of MIPS Technologies, Inc. The information in this document is preliminary and subject to change without notice. MIPS Technologies, Inc. (MTI) reserves the right to change any portion of the product described herein to improve function or design. MTI does not assume liability arising out of the application or use of any product or circuit described herein. Information on MIPS products is available electronically: (a) Through the World Wide Web. Point your WWW client to: http://www.mips.com (b) Through ftp from the internet site “sgigate.sgi.com”. Login as “ftp” or “anonymous” and then cd to the directory “pub/doc”. (c) Through an automated FAX service: Inside the USA toll free: (800) 446-6477 (800-IGO-MIPS) Outside the USA: (415) 688-4321 (call from a FAX machine) MIPS Technologies, Inc. -

2019 NFCA Texas High School Leadoff Classic Main Bracket Results
2019 NFCA Texas High School Leadoff Classic Main Bracket Results Bryan College Station BHS CSHS 1 Bryan College Station 17 Thu 3pm Thu 3pm Cedar Creek BHS CSHS EP Eastlake SA Brandeis 49 Brandeis College Station 57 Cy Woods 1 BHS Thu 5pm Thu 5pm CSHS 2 18 Thu 1pm Brandeis Robinson Thu 1pm EP Montwood BHS CSHS Robinson 11 Huntsville 3 89 Klein Splendora 93 Splendora 9 BHS Fri 2pm Fri 2pm CSHS 3 Fredericksburg Splendora 19 Thu 9am Thu 9am Fredericksburg 6 VET1 VET2 Belton 6 Grapevine 50 58 Richmond Foster 11 BHS Thu 5pm Klein Splendora Thu 5pm CSHS 4 20 Thu 11am Klein Richmond Foster Thu 11am Klein 11 BHS CSHS Rockwall 2 SA Southwest 9 97 SA Southwest Cedar Ridge 99 Cy Ranch 7 VET1 Fri 4pm Fri 4pm CP3 5 Southwest Cy Ranch 21 Thu 11am Thu 1pm Temple 5 VET1 CP3 Plano East 5 Clear Springs 2 51 Southwest Alvin 59 Alvin VET1 Thu 3pm Thu 5pm CP3 6 22 Thu 9am Vandegrift Alvin Thu 3pm Vandegrift 5 BHS CSHS Lufkin San Marcos 5 90 94 Flower Mound 0 VET2 Fri 12pm Southwest Cedar Ridge Fri 12pm CP4 7 San Marcos Cedar Ridge 23 Thu 9am Thu 1pm Magnolia West 3 VET2 CHAMPIONSHIP CP4 RR Cedar Ridge 12 McKinney Boyd 3 52 Game 192 60 SA Johnson VET2 Thu 3pm San Marcos BHS Cedar Ridge Thu 5 pm CP4 8 4:00 PM 24 Thu 11am M. Boyd BHS 5 10 BHS Johnson Thu 3pm Manvel 0 129 Southwest vs. Cedar Ridge 130 Kingwood Park Bellaire 3 Sat 10am Sat 8am Deer Park VET4 BRAC-BB 9 Cedar Park Deer Park 25 Thu 11am Thu 1pm Cedar Park 5 VET4 BRAC-BB Leander Clements 13 53 Cedar Park MacArthur 61 SA MacArthur VET4 Thu 3pm Thu 5pm BRAC-BB 10 26 Thu 9am Clements MacArthur Thu 3pm Waco University 0 BHS CSHS Tomball Memorial Cy Fair 4 91 Friendswood Woodlands 95 Woodlands 2 VET5 Fri 8am Fri 8am BRAC-YS 11 San Benito Woodlands 27 Thu 11am Thu 1pm San Benito 10 VET5 BRAC-YS SA Holmes 1 Friendswood 10 54 62 Santa Fe VET5 Thu 3pm Friendswood Woodlands Thu 5pm BRAC-YS 12 28 Thu 9am Friendswood Santa Fe Thu 3pm Henderson 0 VET1 VET2 Lake Travis Ridge Point 16 98 100 B. -

Classification Made Easy Class 1
Classification Made Easy Class 1 (CP1) The most severely disabled athletes belong to this classification. These athletes are dependent on a power wheelchair or assistance for mobility. They have severe limitation in both the arms and the legs and have very poor trunk control. Sports Available: • Race Runner (RR1) – using the Race Runner frame to run, track events include 100m, 200m and 400m. • Boccia o Boccia Class 1 (BC1) – players who fit into this category can throw the ball onto the court or a CP2 Lower who chooses to push the ball with the foot. Each BC1 athlete has a sport assistant on court with them. o Boccia Class 3 (BC3) – players who fit into this category cannot throw the ball onto the court and have no sustained grasp or release action. They will use a “chute” or “ramp” with the help from their sport assistant to propel the ball. They may use head or arm pointers to hold and release the ball. Players with a impairment of a non cerebral origin, severely affecting all four limbs, are included in this class. Class 2 (CP2) These athletes have poor strength or control all limbs but are able to propel a wheelchair. Some Class 2 athletes can walk but can never run functionally. The class 2 athletes can throw a ball but demonstrates poor grasp and release. Sports Available: • Race Runner (RR2) - using the Race Runner frame to run, track events include 100m, 200m and 400m. • Boccia o Boccia Class 2 (BC2) – players can throw the ball into the court consistently and do not need on court assistance. -

The US Livestock Industry
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1983 The SU livestock industry: an evaluation of the adequacy and relevance of three models of consumer and producer behavior Stephen Stanley Steyn Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agricultural and Resource Economics Commons, and the Agricultural Economics Commons Recommended Citation Steyn, Stephen Stanley, "The SU livestock industry: an evaluation of the adequacy and relevance of three models of consumer and producer behavior " (1983). Retrospective Theses and Dissertations. 7653. https://lib.dr.iastate.edu/rtd/7653 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. -

Athletics Classification Rules and Regulations 2
IPC ATHLETICS International Paralympic Committee Athletics Classifi cation Rules and Regulations January 2016 O cial IPC Athletics Partner www.paralympic.org/athleticswww.ipc-athletics.org @IPCAthletics ParalympicSport.TV /IPCAthletics Recognition Page IPC Athletics.indd 1 11/12/2013 10:12:43 Purpose and Organisation of these Rules ................................................................................. 4 Purpose ............................................................................................................................... 4 Organisation ........................................................................................................................ 4 1 Article One - Scope and Application .................................................................................. 6 International Classification ................................................................................................... 6 Interpretation, Commencement and Amendment ................................................................. 6 2 Article Two – Classification Personnel .............................................................................. 8 Classification Personnel ....................................................................................................... 8 Classifier Competencies, Qualifications and Responsibilities ................................................ 9 3 Article Three - Classification Panels ................................................................................ 11 4 Article Four -

An Advanced System of the Mitochondrial Processing Peptidase and Core Protein Family in Trypanosoma Brucei and Multiple Origins of the Core I Subunit in Eukaryotes
GBE An Advanced System of the Mitochondrial Processing Peptidase and Core Protein Family in Trypanosoma brucei and Multiple Origins of the Core I Subunit in Eukaryotes Jan Mach1, Pavel Poliak2,3,AnnaMatusˇkova´ 4,Vojteˇch Zˇa´rsky´1,Jirˇı´ Janata4, Julius Lukesˇ2,3,and Jan Tachezy1,* 1Department of Parasitology, Faculty of Science, Charles University, Prague, Czech Republic 2Institute of Parasitology, Biology Centre, Czech Academy of Sciences, Prague, Czech Republic 3Faculty of Science, University of South Bohemia, Cˇ eske´ Budeˇjovice, Budweis, Czech Republic 4Institute of Microbiology, Czech Academy of Sciences, Prague, Czech Republic *Corresponding author: E-mail: [email protected]. Accepted: April 2, 2013 Abstract Mitochondrial processing peptidase (MPP) consists of a and b subunits that catalyze the cleavage of N-terminal mitochondrial- targeting sequences (N-MTSs) and deliver preproteins to the mitochondria. In plants, both MPP subunits are associated with the respiratory complex bc1, which has been proposed to represent an ancestral form. Subsequent duplication of MPP subunits resulted in separate sets of genes encoding soluble MPP in the matrix and core proteins (cp1 and cp2) of the membrane-embedded bc1 complex. As only a-MPP was duplicated in Neurospora,itssingleb–MPP functions in both MPP and bc1 complexes. Herein, we investigated the MPP/core protein family and N-MTSs in the kinetoplastid Trypanosoma brucei, which is often considered one of the most ancient eukaryotes. Analysis of N-MTSs predicted in 336 mitochondrial proteins showed that trypanosomal N-MTSs were comparable with N-MTSs from other organisms. N-MTS cleavage is mediated by a standard heterodimeric MPP, which is present in the matrix of procyclic and bloodstream trypanosomes, and its expression is essential for the parasite. -

Technology Enhancement and Ethics in the Paralympic Games
UNIVERSITY OF PELOPONNESE FACULTY OF HUMAN MOVEMENT AND QUALITY OF LIFE SCIENCES DEPARTMENT OF SPORTS ORGANIZATION AND MANAGEMENT MASTER’S THESIS “OLYMPIC STUDIES, OLYMPIC EDUCATION, ORGANIZATION AND MANAGEMENT OF OLYMPIC EVENTS” Technology Enhancement and Ethics In the Paralympic Games Stavroula Bourna Sparta 2016 i TECHNOLOGY ENHANCEMENT AND ETHICS IN THE PARALYMPIC GAMES By Stavroula Bourna MASTER Thesis submitted to the professorial body for the partial fulfillment of obligations for the awarding of a post-graduate title in the Post-graduate Programme, "Organization and Management of Olympic Events" of the University of the Peloponnese, in the branch "Olympic Education" Sparta 2016 Approved by the Professor body: 1st Supervisor: Konstantinos Georgiadis Prof. UNIVERSITY OF PELOPONNESE, GREECE 2nd Supervisor: Konstantinos Mountakis Prof. UNIVERSITY OF PELOPONNESE, GREECE 3rd Supervisor: Paraskevi Lioumpi, Prof., GREECE ii Copyright © Stavroula Bourna, 2016 All rights reserved. The copying, storage and forwarding of the present work, either complete or in part, for commercial profit, is forbidden. The copying, storage and forwarding for non profit-making, educational or research purposes is allowed under the condition that the source of this information must be mentioned and the present stipulations be adhered to. Requests concerning the use of this work for profit-making purposes must be addressed to the author. The views and conclusions expressed in the present work are those of the writer and should not be interpreted as representing the official views of the Department of Sports’ Organization and Management of the University of the Peloponnese. iii ABSTRACT Stavroula Bourna: Technology Enhancement and Ethics in the Paralympic Games (Under the supervision of Konstantinos Georgiadis, Professor) The aim of the present thesis is to present how the new technological advances can affect the performance of the athletes in the Paralympic Games. -

Generalized CP Symmetries and Special Regions of Parameter Space in the Two-Higgs-Doublet Model
PHYSICAL REVIEW D 79, 116004 (2009) Generalized CP symmetries and special regions of parameter space in the two-Higgs-doublet model P.M. Ferreira,1,2 Howard E. Haber,3 and Joa˜o P. Silva1,4 1Instituto Superior de Engenharia de Lisboa, Rua Conselheiro Emı´dio Navarro, 1900 Lisboa, Portugal 2Centro de Fı´sica Teo´rica e Computacional, Faculdade de Cieˆncias, Universidade de Lisboa, Av. Prof. Gama Pinto 2, 1649-003 Lisboa, Portugal 3Santa Cruz Institute for Particle Physics, University of California, Santa Cruz, California 95064, USA 4Centro de Fı´sica Teo´rica de Partı´culas, Instituto Superior Te´cnico, P-1049-001 Lisboa, Portugal (Received 27 March 2009; published 15 June 2009) We consider the impact of imposing generalized CP symmetries on the Higgs sector of the two-Higgs- doublet model, and identify three classes of symmetries. Two of these classes constrain the scalar potential parameters to an exceptional region of parameter space, which respects either a Z2 discrete flavor symmetry or a Uð1Þ symmetry. We exhibit a basis-invariant quantity that distinguishes between these two possible symmetries. We also show that the consequences of imposing these two classes of CP symmetry can be achieved by combining Higgs family symmetries, and that this is not possible for the usual CP symmetry. We comment on the vacuum structure and on renormalization in the presence of these symmetries. Finally, we demonstrate that the standard CP symmetry can be used to build all the models we identify, including those based on Higgs family symmetries. DOI: 10.1103/PhysRevD.79.116004 PACS numbers: 11.30.Er, 11.30.Ly, 12.60.Fr, 14.80.Cp metry. -

PD-1 Expression by Macrophages Plays a Pathologic Role in Altering Microbial Clearance and the Innate Inflammatory Response to Sepsis
PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis Xin Huanga, Fabienne Veneta, Yvonne L. Wanga, Alain Lepapeb, Zhenglong Yuana, Yaping Chena, Ryan Swana, Hakim Kheroufb, Guillaume Monneretb, Chun-Shiang Chunga, and Alfred Ayalaa,1 aDivision of Surgical Research, Department of Surgery, Brown University School of Medicine/Rhode Island Hospital, 593 Eddy Street, Providence, RI 02903; and bImmunology Laboratory, Hospices Civils de Lyon, 69437 Lyon Cedex 03, France Edited by Peter A. Ward, University of Michigan Medical School, Ann Arbor, MI, and accepted by the Editorial Board February 24, 2009 (received for review October 8, 2008) Sepsis, a leading cause of death worldwide, involves concomitant or inhibitory, are critical to a balanced immune response (11). expression of an overzealous inflammatory response and ineffi- Distinguishing it from other members of the CD28 family, pro- cient bacterial clearance. Macrophage function is pivotal to the grammed death 1 (PDF-1) is widely expressed in tissues and organs development of these two aspects during sepsis; however, the and participates in a larger spectrum of immune responses. Studies mechanisms underlying these changes remain unclear. Here we have shown that PD-1 is inducibly expressed on CD4ϩ T cells, report that the PD-1:PD-L pathway appears to be a determining CD8ϩ T cells, NK T cells, B cells, and monocytes upon activation factor of the outcome of sepsis, regulating the delicate balance and plays critical roles in the regulation of autoimmunity, tumor between effectiveness and damage by the antimicrobial immune immunity, viral/parasite immunity, transplantation immunity, al- response. -

CP CHEMICAL INDUSTRY PALLETS Edition: 7
July 2017 CP CHEMICAL INDUSTRY PALLETS Edition: 7 Foreword The European chemical and polymer industry is using a large amount of wooden pallets for the distribution of goods. For environmental, quality and safety reasons there is a strong need to organise the use and re-use of these pallets. Within PlasticsEurope, a team of experts from various chemicals and plastics producing companies, together with pallet specialists, have developed in the early ‘90 a standard for wooden pallets and drafted the present manufacturing and reconditioning specifications of the Chemical industry Pallets, called CP pallets. Special attention has been paid to quality, safety and environmental aspects. Although CP pallets have been designed for specific packages commonly used within the chemical and polymer industry, they are also suitable for other loads. Tests performed by various companies and testing institutes and experience from the use and re-use of millions of CP’s since 1991 have demonstrated that they conform to the needs of the chemical and polymer industry and their customers. However, PlasticsEurope Services cannot be held responsible for any problems or liabilities which may result from the use of the CP pallets system. Scope This document describes the pallet supplier registration and the system to collect used CP pallets. It defines the CP manufacturing, reconditioning and quality assurance criteria. It informs about the safe working load of CP’s. Correspondence can be addressed to: Avenue E. Van Nieuwenhuyse 4 Box 3 at 1160 Brussels, Belgium [email protected] www.plasticseurope.org Note: The content of this document is not essentially different from the previous edition, but has been completed with clarifications about the Supplier registration (header A.) and Marking (header B. -

Genetic Background of Ataxia in Children Younger Than 5 Years in Finland E444
Volume 6, Number 4, August 2020 Neurology.org/NG A peer-reviewed clinical and translational neurology open access journal ARTICLE Genetic background of ataxia in children younger than 5 years in Finland e444 ARTICLE Cerebral arteriopathy associated with heterozygous variants in the casitas B-lineage lymphoma gene e448 ARTICLE Somatic SLC35A2 mosaicism correlates with clinical fi ndings in epilepsy brain tissuee460 ARTICLE Synonymous variants associated with Alzheimer disease in multiplex families e450 Academy Officers Neurology® is a registered trademark of the American Academy of Neurology (registration valid in the United States). James C. Stevens, MD, FAAN, President Neurology® Genetics (eISSN 2376-7839) is an open access journal published Orly Avitzur, MD, MBA, FAAN, President Elect online for the American Academy of Neurology, 201 Chicago Avenue, Ann H. Tilton, MD, FAAN, Vice President Minneapolis, MN 55415, by Wolters Kluwer Health, Inc. at 14700 Citicorp Drive, Bldg. 3, Hagerstown, MD 21742. Business offices are located at Two Carlayne E. Jackson, MD, FAAN, Secretary Commerce Square, 2001 Market Street, Philadelphia, PA 19103. Production offices are located at 351 West Camden Street, Baltimore, MD 21201-2436. Janis M. Miyasaki, MD, MEd, FRCPC, FAAN, Treasurer © 2020 American Academy of Neurology. Ralph L. Sacco, MD, MS, FAAN, Past President Neurology® Genetics is an official journal of the American Academy of Neurology. Journal website: Neurology.org/ng, AAN website: AAN.com CEO, American Academy of Neurology Copyright and Permission Information: Please go to the journal website (www.neurology.org/ng) and click the Permissions tab for the relevant Mary E. Post, MBA, CAE article. Alternatively, send an email to [email protected].