Bioerosion and Fungal Colonization of the Invasive Foraminiferan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phaeoseptaceae, Pleosporales) from China
Mycosphere 10(1): 757–775 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/17 Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China Liu NG1,2,3,4,5, Hyde KD4,5, Bhat DJ6, Jumpathong J3 and Liu JK1*,2 1 School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China 2 Guizhou Key Laboratory of Agricultural Biotechnology, Guizhou Academy of Agricultural Sciences, Guiyang 550006, P.R. China 3 Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand 4 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 5 Mushroom Research Foundation, Chiang Rai 57100, Thailand 6 No. 128/1-J, Azad Housing Society, Curca, P.O., Goa Velha 403108, India Liu NG, Hyde KD, Bhat DJ, Jumpathong J, Liu JK 2019 – Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China. Mycosphere 10(1), 757–775, Doi 10.5943/mycosphere/10/1/17 Abstract A new hyphomycete genus, Pleopunctum, is introduced to accommodate two new species, P. ellipsoideum sp. nov. (type species) and P. pseudoellipsoideum sp. nov., collected from decaying wood in Guizhou Province, China. The genus is characterized by macronematous, mononematous conidiophores, monoblastic conidiogenous cells and muriform, oval to ellipsoidal conidia often with a hyaline, elliptical to globose basal cell. Phylogenetic analyses of combined LSU, SSU, ITS and TEF1α sequence data of 55 taxa were carried out to infer their phylogenetic relationships. The new taxa formed a well-supported subclade in the family Phaeoseptaceae and basal to Lignosphaeria and Thyridaria macrostomoides. -

Elsevier Editorial System(Tm) for Fungal Ecology Manuscript Draft
Elsevier Editorial System(tm) for Fungal Ecology Manuscript Draft Manuscript Number: FUNECO-D-16-00203R1 Title: Intra-diurnal and daily changes in Didymella ascospore concentrations in the air of an urban site Article Type: Original Research Paper Keywords: pathogen; bioaerosol; asthma; exposure time; air quality; fungal ecology Corresponding Author: Dr. Magdalena Sadyś, Ph.D. Corresponding Author's Institution: Rothamsted Research First Author: Magdalena Sadyś, Ph.D. Order of Authors: Magdalena Sadyś, Ph.D.; Jonathan S West, Ph.D. Abstract: Didymella sp. is a common plant pathogen affecting mainly cereal crops in countries with temperate climates, and its airborne spores are also a potential human allergen. A 5-year monitoring study was carried out at an urban site in the UK to establish the most likely exposure time in order to alert people sensitised to spores of this genus. Didymella ascospores occurred in air with a bimodal pattern, with peak concentrations occurring at 03:00 and 22:00. The majority of ascospores were observed from 20:30 to 07:30 according to a multivariate regression tree analysis. Similarly, circular tests indicated that the maximum hourly concentrations were found in the morning hours. The highest ascospore concentrations were observed in very humid conditions occurring after rainfall. The observations taken from an urban site were delayed in relation to the time of ascospore release previously reported from field sites. Thus, there is a high possibility of regional transport of ascospores in the atmosphere from remote sources. Cover Letter Intra-diurnal and daily changes in Didymella ascospore concentrations in the air of an urban site Magdalena Sadyś 1,2 , Jonathan S. -

Taxonomy and Multigene Phylogenetic Evaluation of Novel Species in Boeremia and Epicoccum with New Records of Ascochyta and Didymella (Didymellaceae)
Mycosphere 8(8): 1080–1101 (2017) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/8/8/9 Copyright © Guizhou Academy of Agricultural Sciences Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae) Jayasiri SC1,2, Hyde KD2,3, Jones EBG4, Jeewon R5, Ariyawansa HA6, Bhat JD7, Camporesi E8 and Kang JC1 1 Engineering and Research Center for Southwest Bio-Pharmaceutical Resources of National Education Ministry of China, Guizhou University, Guiyang, Guizhou Province 550025, P.R. China 2Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 3World Agro forestry Centre East and Central Asia Office, 132 Lanhei Road, Kunming 650201, P. R. China 4Botany and Microbiology Department, College of Science, King Saud University, Riyadh, 1145, Saudi Arabia 5Department of Health Sciences, Faculty of Science, University of Mauritius, Reduit, Mauritius 6Department of Plant Pathology and Microbiology, College of BioResources and Agriculture, National Taiwan University, No.1, Sec.4, Roosevelt Road, Taipei 106, Taiwan, ROC. 7No. 128/1-J, Azad Housing Society, Curca, P.O. Goa Velha, 403108, India 89A.M.B. Gruppo Micologico Forlivese “Antonio Cicognani”, Via Roma 18, Forlì, Italy; A.M.B. CircoloMicologico “Giovanni Carini”, C.P. 314, Brescia, Italy; Società per gliStudiNaturalisticidella Romagna, C.P. 144, Bagnacavallo (RA), Italy *Correspondence: [email protected] Jayasiri SC, Hyde KD, Jones EBG, Jeewon R, Ariyawansa HA, Bhat JD, Camporesi E, Kang JC 2017 – Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae). -

Ecology and Taxonomy of Leptosphaerulina Spp. Associated with Turfgrasses in the United States
Ecology and Taxonomy of Leptosphaerulina spp. Associated with Turfgrasses in the United States Steven W. Abler Thesis submitted to the Faculty of the Virginia Polytechnic Institute and State University In partial fulfillment of the requirements for the degree of Master of Science in Plant Pathology, Physiology, and Weed Science H.B. Couch, Chair A.B. Baudoin E.H. Ervin January 31, 2003 Blacksburg, Virginia Key Words: Leptosphaerulina, Turfgrass, Morphology, Phylogenetics, ITS, EF-1α Copyright 2003, Steven W. Abler Ecology and Taxonomy of Leptosphaerulina spp. Associated with Turfgrasses in the United States Steve W. Abler Abstract Leptosphaerulina spp. are common fungi that have been reported to colonize several turfgrass species. Controversy exists regarding the relationship of Leptosphaerulina spp. and their turfgrass hosts. The fungus has been classified as a saprophyte, senectophyte, weak pathogen, and pathogen of turfgrasses. There has also been conflicting reports regarding the delineation of species within the genus Leptosphaerulina. Because of the uncertainty regarding the ecology and taxonomy of the genus in relation to turfgrasses the present study was undertaken. The ITS and EF-1α gene regions were sequenced and analyzed to compare to the multiple taxonomic schemes reported in the literature. The ITS region offered no resolution of species; however, the phylogeny of the EF-1α gene was consistent with the six-species model of Graham and Luttrell. Inoculation experiments were performed on unstressed and artificially stressed plants to determine whether the fungi are pathogens, senectophytes, or saprophytes of turfgrasses. Perennial ryegrass and creeping bentgrass plants were stressed by placing them in a dew chamber set at 38ºC, 100% R.H., and no light for two and one days respectively. -

Apple Scab (Venturia Inaequalis) and Pests in Organic Orchards
Apple Scab (Venturia inaequalis) and Pests in Organic Orchards Boel Sandskär Department of Crop Science, Alnarp Doctoral Thesis Swedish University of Agricultural Sciences Alnarp 2003 2 Abstract Sandskär, B. Apple Scab (Venturia inaequalis) and Pests in Organic Orchards Doctoral Dissertation ISSN 1401-6249, ISBN 91-576-6416-1 Domestication of apples goes back several thousand years in time and archaeological findings of dried apples from Östergötland in Sweden have been dated to ca 2 500 B.C. Worldwide, apples are considered an attractive and healthy fruit to eat. Organic production of apples is increasing abroad but is still at very low levels in Sweden. This study deals with major disease and pest problems in organic growing of apples. It concentrates on the most severe disease, the apple scab (Venturia inaequalis). Resistance to apple scab was evaluated during three years in over 450 old and new apple cultivars at Alnarp and Balsgård in southern Sweden. There were significant differences between the cultivars and years. About ten per cent of the cultivars had a high level of resistance against apple scab. The correlation between foliar and fruit scab was stronger when the scab infection pressure was high (1998-1999), compared to when it was low (2000). Polygenic resistance is a desirable trait since such resistance is more difficult to overcome by the pathogen. A common denominator for polygenic resistance among the cultivars assessed was 'Worcester Pearmain'. The leaf infection of apple scab was compared at three locations: Alnarp, Kivik and Rånna (Skövde) in an observation trial for 22 new apple cultivars. The ranking of the cultivars was similar at the three locations. -

Epicoccum Sp., an Emerging Source of Unique Bioactive Metabolites
Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 73 No. 1 pp. 13ñ21, 2016 ISSN 0001-6837 Polish Pharmaceutical Society EPICOCCUM SP., AN EMERGING SOURCE OF UNIQUE BIOACTIVE METABOLITES NIGHAT FATIMA1*, TARIQ ISMAIL1, SYED AUN MUHAMMAD3, MUNIBA JADOON4, SAFIA AHMED4, SAIRA AZHAR1 and AMARA MUMTAZ2* 1Department of Pharmacy, 2Department of Chemistry, COMSATS Institute of Information Technology, Abbottabad, Pakistan, 22060 3Institute of Molecular Biology and Biotechnology, Bahauddin Zakariya University Multan, Pakistan 4Department of Microbiology, Quaid-I-Azam University, Islamabad, 45320, Pakistan Abstract: Fungi are playing a vital role for producing natural products, most productive source of lead com- pounds in far reaching endeavor of new drug discovery. Epicoccum fungus is known for its potential to produce diverse classes of biologically active secondary metabolites. The intent of this review is to provide detailed information about biology and chemistry of Epicoccum fungus. Most of the fungus metabolites showed cyto- toxic, anticancer, antimicrobial and anti-diabetic activities. The literature given encompases the details of iso- lation of different unusual and unique secondary metabolites, their chemical nature and biological activities find out Epicoccum spp., a potential source of lead molecules. Keywords: anticancer, biocontrol, Epicoccum, epicorazines, epicoccamides In the food, cosmetics and pharmaceutical inner tissues of several plant species (7, 8). In plant industries, the fungi are important for their role in pest E. nigrum can be used as a biological control (9- different biotechnological processes like fermenta- 12). Many scientists had focused on study of wide tion, synthesis and production of bioactive metabo- variety of anticancer, antimicrobial and anti-diabet- lites (1). Out of 1.5 million known fungal species, ic metabolites from E. -
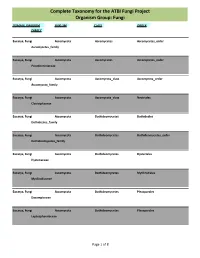
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Ascomycetes_family Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Pseudeurotiaceae Eucarya, Fungi Ascomycota Ascomycota_class Ascomycota_order Ascomycota_family Eucarya, Fungi Ascomycota Ascomycota_class Nectriales Clavicipitaceae Eucarya, Fungi Ascomycota Dothideomycetes Dothideales Dothideales_family Eucarya, Fungi Ascomycota Dothideomycetes Dothideomycetes_order Dothideomycetes_family Eucarya, Fungi Ascomycota Dothideomycetes Hysteriales Hysteriaceae Eucarya, Fungi Ascomycota Dothideomycetes Mytilinidiales Mytilinidiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Dacampiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Leptosphaeriaceae Page 1 of 8 Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Lophiostomataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Massarinaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Melanommataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleomassariaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales_family Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Sporormiaceae Eucarya, Fungi Ascomycota Dothideomycetes -

Necrotrophic Pathogens of Wheat
This article was originally published in the Encyclopedia of Food Grains published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author’s institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial Oliver R.P., Tan K.-C. and Moffat C.S. (2016) Necrotrophic Pathogens of Wheat. In: Wrigley, C., Corke, H., and Seetharaman, K., Faubion, J., (eds.) Encyclopedia of Food Grains, 2nd Edition, pp. 273-278. Oxford: Academic Press. © 2016 Elsevier Ltd. All rights reserved. Author's personal copy Necrotrophic Pathogens of Wheat RPOliver, K-C Tan, and CS Moffat, Curtin University, Bentley, WA, Australia ã 2016 Elsevier Ltd. All rights reserved. Topic Highlights give information of trends over decades (Brennan and Murray, 1988, 1998). Table 2 lists estimates of current wheat disease • Diseases of wheat. losses in Australia. It can be seen that across all regions, TS is • Genetic analysis of resistance to tan spot and Septoria the major disease, currently costing more than all rusts com- nodorum blotch (SNB) necrotrophic effectors. bined. SNB is restricted to Western Australia where it ranks as Tan spot effectors. -

Some Studies on Leaf Spot of Oats and Triticale Mohammed Abdullah Lashram South Dakota State University
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Electronic Theses and Dissertations 2019 Some Studies on Leaf Spot of Oats and Triticale Mohammed Abdullah Lashram South Dakota State University Follow this and additional works at: https://openprairie.sdstate.edu/etd Part of the Agronomy and Crop Sciences Commons, and the Plant Pathology Commons Recommended Citation Lashram, Mohammed Abdullah, "Some Studies on Leaf Spot of Oats and Triticale" (2019). Electronic Theses and Dissertations. 3125. https://openprairie.sdstate.edu/etd/3125 This Thesis - Open Access is brought to you for free and open access by Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. SOME STUDIES ON LEAF SPOT OF OATS AND TRITICALE BY MOHAMMED ABDULLAH LASHRAM A thesis submitted in partial fulfillment of the requirements for the Master of Science Major in Plant Science South Dakota State University 2019 iii ACKNOWLEDGEMENTS First and foremost, I wish to pay my sincere regards to the Almighty, who provided me with an opportunity, to begin with, my research for my MS and making me conduct it successfully. My most heartfelt thanks to my advisor Dr. Shaukat Ali for providing me a platform for working on this project as a master thesis. He has been my source of inspiration and encouragement throughout my stay at SDSU. I especially want to thank Dr. -

Hidden Fungi: Combining Culture-Dependent and -Independent DNA Barcoding Reveals Inter-Plant Variation in Species Richness of Endophytic Root Fungi in Elymus Repens
Journal of Fungi Article Hidden Fungi: Combining Culture-Dependent and -Independent DNA Barcoding Reveals Inter-Plant Variation in Species Richness of Endophytic Root Fungi in Elymus repens Anna K. Høyer and Trevor R. Hodkinson * Botany, School of Natural Sciences, Trinity College Dublin, The University of Dublin, Dublin D2, Ireland; [email protected] * Correspondence: [email protected] Abstract: The root endophyte community of the grass species Elymus repens was investigated using both a culture-dependent approach and a direct amplicon sequencing method across five sites and from individual plants. There was much heterogeneity across the five sites and among individual plants. Focusing on one site, 349 OTUs were identified by direct amplicon sequencing but only 66 OTUs were cultured. The two approaches shared ten OTUs and the majority of cultured endo- phytes do not overlap with the amplicon dataset. Media influenced the cultured species richness and without the inclusion of 2% MEA and full-strength MEA, approximately half of the unique OTUs would not have been isolated using only PDA. Combining both culture-dependent and -independent methods for the most accurate determination of root fungal species richness is therefore recom- mended. High inter-plant variation in fungal species richness was demonstrated, which highlights the need to rethink the scale at which we describe endophyte communities. Citation: Høyer, A.K.; Hodkinson, T.R. Hidden Fungi: Combining Culture-Dependent and -Independent Keywords: DNA barcoding; Elymus repens; fungal root endophytes; high-throughput amplicon DNA Barcoding Reveals Inter-Plant sequencing; MEA; PDA Variation in Species Richness of Endophytic Root Fungi in Elymus repens. J. Fungi 2021, 7, 466. -

The Family Pyrenidiaceae Resurrected
Mycosphere 10(1): 634–654 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/13 The family Pyrenidiaceae resurrected Huanraluek N1, Ertz D3,5, Phukhamsakda C1, Hongsanan S2, Jayawardena RS1 and Hyde KD1,4* 1Center of Excellence in Fungal Research, and School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand 2Shenzhen Key Laboratory of Microbial Genetic Engineering, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen 518060, China 3Department Research, Meise Botanic Garden, BE-1860 Meise, Belgium 4Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, China 5Fédération Wallonie-Bruxelles, Direction Générale de l′Enseignement non obligatorire et de la Recherche scientifique, Rue A. Lavallée 1, B-1080 Bruxelles, Belgium Huanraluek N, Ertz D, Phukhamsakda C, Hongsanan S, Jayawardena RS, Hyde KD 2019 – The family Pyrenidiaceae resurrected. Mycosphere 10(1), 634–654, Doi 10.5943/mycosphere/10/1/13 Abstract Pyrenidium is a lichenicolous genus which was included in the family Dacampiaceae (Pleosporales) based on morphological characters. The classification of this genus within Dacampiaceae has been controversial due to the lack of sequence data. In this study, the genus Pyrenidium is sequenced for the first time using five freshly collected specimens belonging to the generic type and two other species. Although the morphology of Pyrenidium is quite similar to other genera of Dacampiaceae, phylogenetic analyses from nuLSU and nuSSU sequence data demonstrate that Pyrenidium is distantly related to Dacampiaceae and it forms a distinct lineage within the Dothideomycetes. Therefore, we resurrect the family Pyrenidiaceae to accommodate Pyrenidium. Morphological descriptions of the sequenced specimens of Pyrenidium are provided and include the description of a new species, P. -

Pleosporales, Dothideomycetes)
Mycosphere 5 (3): 411–417 (2014) ISSN 2077 7019 www.mycosphere.org Article Mycosphere Copyright © 2014 Online Edition Doi 10.5943/mycosphere/5/3/3 A new species, Lophiostoma versicolor, from Japan (Pleosporales, Dothideomycetes) Hirayama K1, Hashimoto A2, 3 and Tanaka K2 1 Apple Experiment Station, Aomori Prefectural Agriculture and Forestry Research Center, 24 Fukutami, Botandaira, Kuroishi, Aomori 036-0332, Japan 2 Faculty of Agriculture and Life Sciences, Hirosaki University, 3 Bunkyo-cho, Hirosaki, Aomori, 036-8561, Japan 3The United Graduate School of Agricultural Sciences, Iwate University, 18-8 Ueda 3 chome, Morioka 020-8550, Japan Hirayama K, Hashimoto A, Tanaka K 2014 – A new species, Lophiostoma versicolor, from Japan (Pleosporales, Dothideomycetes). Mycosphere 5(3), 411–417, Doi 10.5943/mycosphere/5/3/3 Abstract Lophiostoma versicolor sp. nov. was found on Acer sp. in Japan. This species is characterized by ascomata with a laterally compressed apex; clavate, 2(–4)-spored asci with a long stipe; and verruculose, 3-septate, versicolored ascospores without a sheath or appendages. Phylogenetic analyses based on LSU nrDNA sequences supported the generic placement and species validity of L. versicolor. Key words – ITS – Lophiostomataceae – Lophiotrema – LSU nrDNA – Pleosporomycetidae – Systematics – Taxonomy Introduction During an investigation of bitunicate ascomycetes in Japan, an unidentified fungus was found on dead twigs of Acer sp. The morphological characteristics of the fungus, such as the presence of ascomata with a compressed beak and clavate asci, recall those of Lophiostoma (Hirayama & Tanaka 2011) belonging to the Lophiostomataceae. This fungus, however, is different from any of the existing species of the genus because it possesses 2(–4)-spored asci and verruculose, 3-septate, versicolored ascospores without a sheath or appendages.