Epicoccum Sp., an Emerging Source of Unique Bioactive Metabolites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phaeoseptaceae, Pleosporales) from China
Mycosphere 10(1): 757–775 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/17 Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China Liu NG1,2,3,4,5, Hyde KD4,5, Bhat DJ6, Jumpathong J3 and Liu JK1*,2 1 School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China 2 Guizhou Key Laboratory of Agricultural Biotechnology, Guizhou Academy of Agricultural Sciences, Guiyang 550006, P.R. China 3 Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand 4 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 5 Mushroom Research Foundation, Chiang Rai 57100, Thailand 6 No. 128/1-J, Azad Housing Society, Curca, P.O., Goa Velha 403108, India Liu NG, Hyde KD, Bhat DJ, Jumpathong J, Liu JK 2019 – Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China. Mycosphere 10(1), 757–775, Doi 10.5943/mycosphere/10/1/17 Abstract A new hyphomycete genus, Pleopunctum, is introduced to accommodate two new species, P. ellipsoideum sp. nov. (type species) and P. pseudoellipsoideum sp. nov., collected from decaying wood in Guizhou Province, China. The genus is characterized by macronematous, mononematous conidiophores, monoblastic conidiogenous cells and muriform, oval to ellipsoidal conidia often with a hyaline, elliptical to globose basal cell. Phylogenetic analyses of combined LSU, SSU, ITS and TEF1α sequence data of 55 taxa were carried out to infer their phylogenetic relationships. The new taxa formed a well-supported subclade in the family Phaeoseptaceae and basal to Lignosphaeria and Thyridaria macrostomoides. -

1 Etiology, Epidemiology and Management of Fruit Rot Of
Etiology, Epidemiology and Management of Fruit Rot of Deciduous Holly in U.S. Nursery Production Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Shan Lin Graduate Program in Plant Pathology The Ohio State University 2018 Dissertation Committee Dr. Francesca Peduto Hand, Advisor Dr. Anne E. Dorrance Dr. Laurence V. Madden Dr. Sally A. Miller 1 Copyrighted by Shan Lin 2018 2 Abstract Cut branches of deciduous holly (Ilex spp.) carrying shiny and colorful fruit are popularly used for holiday decorations in the United States. Since 2012, an emerging disease causing the fruit to rot was observed across Midwestern and Eastern U.S. nurseries. A variety of other symptoms were associated with the disease, including undersized, shriveled, and dull fruit, as well as leaf spots and early plant defoliation. The disease causal agents were identified by laboratory processing of symptomatic fruit collected from nine locations across four states over five years by means of morphological characterization, multi-locus phylogenetic analyses and pathogenicity assays. Alternaria alternata and a newly described species, Diaporthe ilicicola sp. nov., were identified as the primary pathogens associated with the disease, and A. arborescens, Colletotrichum fioriniae, C. nymphaeae, Epicoccum nigrum and species in the D. eres species complex were identified as minor pathogens in this disease complex. To determine the sources of pathogen inoculum in holly fields, and the growth stages of host susceptibility to fungal infections, we monitored the presence of these pathogens in different plant tissues (i.e., dormant twigs, mummified fruit, leaves and fruit), and we studied inoculum dynamics and assessed disease progression throughout the growing season in three Ohio nurseries exposed to natural inoculum over two consecutive years. -

DNA Barcoding of Fungi in the Forest Ecosystem of the Psunj and Papukissn Mountains 1847-6481 in Croatia Eissn 1849-0891
DNA Barcoding of Fungi in the Forest Ecosystem of the Psunj and PapukISSN Mountains 1847-6481 in Croatia eISSN 1849-0891 OrIGINAL SCIENtIFIC PAPEr DOI: https://doi.org/10.15177/seefor.20-17 DNA barcoding of Fungi in the Forest Ecosystem of the Psunj and Papuk Mountains in Croatia Nevenka Ćelepirović1,*, Sanja Novak Agbaba2, Monika Karija Vlahović3 (1) Croatian Forest Research Institute, Division of Genetics, Forest Tree Breeding and Citation: Ćelepirović N, Novak Agbaba S, Seed Science, Cvjetno naselje 41, HR-10450 Jastrebarsko, Croatia; (2) Croatian Forest Karija Vlahović M, 2020. DNA Barcoding Research Institute, Division of Forest Protection and Game Management, Cvjetno naselje of Fungi in the Forest Ecosystem of the 41, HR-10450 Jastrebarsko; (3) University of Zagreb, School of Medicine, Department of Psunj and Papuk Mountains in Croatia. forensic medicine and criminology, DNA Laboratory, HR-10000 Zagreb, Croatia. South-east Eur for 11(2): early view. https://doi.org/10.15177/seefor.20-17. * Correspondence: e-mail: [email protected] received: 21 Jul 2020; revised: 10 Nov 2020; Accepted: 18 Nov 2020; Published online: 7 Dec 2020 AbStract The saprotrophic, endophytic, and parasitic fungi were detected from the samples collected in the forest of the management unit East Psunj and Papuk Nature Park in Croatia. The disease symptoms, the morphology of fruiting bodies and fungal culture, and DNA barcoding were combined for determining the fungi at the genus or species level. DNA barcoding is a standardized and automated identification of species based on recognition of highly variable DNA sequences. DNA barcoding has a wide application in the diagnostic purpose of fungi in biological specimens. -

Elsevier Editorial System(Tm) for Fungal Ecology Manuscript Draft
Elsevier Editorial System(tm) for Fungal Ecology Manuscript Draft Manuscript Number: FUNECO-D-16-00203R1 Title: Intra-diurnal and daily changes in Didymella ascospore concentrations in the air of an urban site Article Type: Original Research Paper Keywords: pathogen; bioaerosol; asthma; exposure time; air quality; fungal ecology Corresponding Author: Dr. Magdalena Sadyś, Ph.D. Corresponding Author's Institution: Rothamsted Research First Author: Magdalena Sadyś, Ph.D. Order of Authors: Magdalena Sadyś, Ph.D.; Jonathan S West, Ph.D. Abstract: Didymella sp. is a common plant pathogen affecting mainly cereal crops in countries with temperate climates, and its airborne spores are also a potential human allergen. A 5-year monitoring study was carried out at an urban site in the UK to establish the most likely exposure time in order to alert people sensitised to spores of this genus. Didymella ascospores occurred in air with a bimodal pattern, with peak concentrations occurring at 03:00 and 22:00. The majority of ascospores were observed from 20:30 to 07:30 according to a multivariate regression tree analysis. Similarly, circular tests indicated that the maximum hourly concentrations were found in the morning hours. The highest ascospore concentrations were observed in very humid conditions occurring after rainfall. The observations taken from an urban site were delayed in relation to the time of ascospore release previously reported from field sites. Thus, there is a high possibility of regional transport of ascospores in the atmosphere from remote sources. Cover Letter Intra-diurnal and daily changes in Didymella ascospore concentrations in the air of an urban site Magdalena Sadyś 1,2 , Jonathan S. -

Taxonomy and Multigene Phylogenetic Evaluation of Novel Species in Boeremia and Epicoccum with New Records of Ascochyta and Didymella (Didymellaceae)
Mycosphere 8(8): 1080–1101 (2017) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/8/8/9 Copyright © Guizhou Academy of Agricultural Sciences Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae) Jayasiri SC1,2, Hyde KD2,3, Jones EBG4, Jeewon R5, Ariyawansa HA6, Bhat JD7, Camporesi E8 and Kang JC1 1 Engineering and Research Center for Southwest Bio-Pharmaceutical Resources of National Education Ministry of China, Guizhou University, Guiyang, Guizhou Province 550025, P.R. China 2Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 3World Agro forestry Centre East and Central Asia Office, 132 Lanhei Road, Kunming 650201, P. R. China 4Botany and Microbiology Department, College of Science, King Saud University, Riyadh, 1145, Saudi Arabia 5Department of Health Sciences, Faculty of Science, University of Mauritius, Reduit, Mauritius 6Department of Plant Pathology and Microbiology, College of BioResources and Agriculture, National Taiwan University, No.1, Sec.4, Roosevelt Road, Taipei 106, Taiwan, ROC. 7No. 128/1-J, Azad Housing Society, Curca, P.O. Goa Velha, 403108, India 89A.M.B. Gruppo Micologico Forlivese “Antonio Cicognani”, Via Roma 18, Forlì, Italy; A.M.B. CircoloMicologico “Giovanni Carini”, C.P. 314, Brescia, Italy; Società per gliStudiNaturalisticidella Romagna, C.P. 144, Bagnacavallo (RA), Italy *Correspondence: [email protected] Jayasiri SC, Hyde KD, Jones EBG, Jeewon R, Ariyawansa HA, Bhat JD, Camporesi E, Kang JC 2017 – Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae). -

Ecology and Taxonomy of Leptosphaerulina Spp. Associated with Turfgrasses in the United States
Ecology and Taxonomy of Leptosphaerulina spp. Associated with Turfgrasses in the United States Steven W. Abler Thesis submitted to the Faculty of the Virginia Polytechnic Institute and State University In partial fulfillment of the requirements for the degree of Master of Science in Plant Pathology, Physiology, and Weed Science H.B. Couch, Chair A.B. Baudoin E.H. Ervin January 31, 2003 Blacksburg, Virginia Key Words: Leptosphaerulina, Turfgrass, Morphology, Phylogenetics, ITS, EF-1α Copyright 2003, Steven W. Abler Ecology and Taxonomy of Leptosphaerulina spp. Associated with Turfgrasses in the United States Steve W. Abler Abstract Leptosphaerulina spp. are common fungi that have been reported to colonize several turfgrass species. Controversy exists regarding the relationship of Leptosphaerulina spp. and their turfgrass hosts. The fungus has been classified as a saprophyte, senectophyte, weak pathogen, and pathogen of turfgrasses. There has also been conflicting reports regarding the delineation of species within the genus Leptosphaerulina. Because of the uncertainty regarding the ecology and taxonomy of the genus in relation to turfgrasses the present study was undertaken. The ITS and EF-1α gene regions were sequenced and analyzed to compare to the multiple taxonomic schemes reported in the literature. The ITS region offered no resolution of species; however, the phylogeny of the EF-1α gene was consistent with the six-species model of Graham and Luttrell. Inoculation experiments were performed on unstressed and artificially stressed plants to determine whether the fungi are pathogens, senectophytes, or saprophytes of turfgrasses. Perennial ryegrass and creeping bentgrass plants were stressed by placing them in a dew chamber set at 38ºC, 100% R.H., and no light for two and one days respectively. -

Apple Scab (Venturia Inaequalis) and Pests in Organic Orchards
Apple Scab (Venturia inaequalis) and Pests in Organic Orchards Boel Sandskär Department of Crop Science, Alnarp Doctoral Thesis Swedish University of Agricultural Sciences Alnarp 2003 2 Abstract Sandskär, B. Apple Scab (Venturia inaequalis) and Pests in Organic Orchards Doctoral Dissertation ISSN 1401-6249, ISBN 91-576-6416-1 Domestication of apples goes back several thousand years in time and archaeological findings of dried apples from Östergötland in Sweden have been dated to ca 2 500 B.C. Worldwide, apples are considered an attractive and healthy fruit to eat. Organic production of apples is increasing abroad but is still at very low levels in Sweden. This study deals with major disease and pest problems in organic growing of apples. It concentrates on the most severe disease, the apple scab (Venturia inaequalis). Resistance to apple scab was evaluated during three years in over 450 old and new apple cultivars at Alnarp and Balsgård in southern Sweden. There were significant differences between the cultivars and years. About ten per cent of the cultivars had a high level of resistance against apple scab. The correlation between foliar and fruit scab was stronger when the scab infection pressure was high (1998-1999), compared to when it was low (2000). Polygenic resistance is a desirable trait since such resistance is more difficult to overcome by the pathogen. A common denominator for polygenic resistance among the cultivars assessed was 'Worcester Pearmain'. The leaf infection of apple scab was compared at three locations: Alnarp, Kivik and Rånna (Skövde) in an observation trial for 22 new apple cultivars. The ranking of the cultivars was similar at the three locations. -

Epipyrone A, a Broad-Spectrum Antifungal Compound Produced by Epicoccum Nigrum ICMP 19927
molecules Article Epipyrone A, a Broad-Spectrum Antifungal Compound Produced by Epicoccum nigrum ICMP 19927 Alex J. Lee 1 , Melissa M. Cadelis 2,3 , Sang H. Kim 1, Simon Swift 3 , Brent R. Copp 2 and Silas G. Villas-Boas 1,* 1 School of Biological Sciences, University of Auckland, 3A Symonds Street, 1010 Auckland, New Zealand; [email protected] (A.J.L.); [email protected] (S.H.K.) 2 School of Chemical Sciences, University of Auckland, 23 Symonds Street, 1010 Auckland, New Zealand; [email protected] (M.M.C.); [email protected] (B.R.C.) 3 School of Medical Sciences, University of Auckland, 85 Park Road, Grafton, 1023 Auckland, New Zealand; [email protected] * Correspondence: [email protected] Academic Editor: Rosa Durán Patron Received: 31 October 2020; Accepted: 16 December 2020; Published: 18 December 2020 Abstract: We have isolated a filamentous fungus that actively secretes a pigmented exudate when growing on agar plates. The fungus was identified as being a strain of Epicoccum nigrum. The fungal exudate presented strong antifungal activity against both yeasts and filamentous fungi, and inhibited the germination of fungal spores. The chemical characterization of the exudate showed that the pigmented molecule presenting antifungal activity is the disalt of epipyrone A—a water-soluble polyene metabolite with a molecular mass of 612.29 and maximal UV–Vis absorbance at 428 nm. This antifungal compound showed excellent stability to different temperatures and neutral to alkaline pH. Keywords: polyenes; pigment; fungicide; antimicrobial; yeast; antibiotic; bioactives 1. Introduction Epicoccum species are primarily saprophytic fungi from the family Didymellaceae. -

Epicoccum Species As Potent Factories for the Production of Compounds of Industrial, Medical, and Biological Control Applications
Review Article ISSN: 2574 -1241 DOI: 10.26717.BJSTR.2019.14.002541 Epicoccum Species as Potent Factories for the Production of Compounds of Industrial, Medical, and Biological Control Applications Waill A Elkhateeb and Ghoson M Daba* Department of Chemistry of Microbial Natural Products, National Research Center, Egypt *Corresponding author: Ghoson Mosbah Daba, Department of Chemistry of Microbial Natural Products, National Research Center, Egypt ARTICLE INFO abstract Received: January 31, 2019 Epicoccum is an endophytic fungus famous for its application in the biocontrol of nu- Published: February 11, 2019 merous phytopathogenic fungi. Moreover, Epicoccum Sp. are known for their capability of producing various biologically active compounds with medical applications as antiox- idant, antimicrobial, and anticancer agents. In addition to pigments formation and their Citation: Ghoson Mosbah Daba. Ep- industrial application. The aim of this review is to highlight the diversity of compounds icoccum Species as Potent Factories produced by Epicoccum sp. and pointing out their medical, bio-control, and industrial ap- for the Production of Compounds plications. of Industrial, Medical, and Biologi- ; Biological Control; Biotechnology; Secondary Metabolites cal Control Applications. Biomed J Keywords: Epicoccum Sci & Tech Res 14(3)-2019. BJSTR. MS.ID.002541. Introduction of biologically active compounds can contribute in encouraging Discovering new applications for currently known bioactive searching for novel sources of potent compounds to face current metabolites and/ or exploring novel biologically active metabolites needs for antimicrobial agents to overcome microbial antibiotic are of critical need nowadays due to the current increasing dilemma resistance, and to discover drugs for existing life-threating diseases. of microbial resistance to available and used antibiotics and therapeutic agents, beside the emergence of new life threatening Secondary Metabolites of Epicoccum Species diseases. -
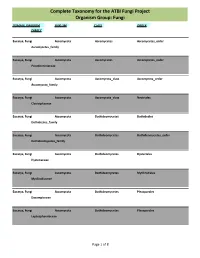
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Ascomycetes_family Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Pseudeurotiaceae Eucarya, Fungi Ascomycota Ascomycota_class Ascomycota_order Ascomycota_family Eucarya, Fungi Ascomycota Ascomycota_class Nectriales Clavicipitaceae Eucarya, Fungi Ascomycota Dothideomycetes Dothideales Dothideales_family Eucarya, Fungi Ascomycota Dothideomycetes Dothideomycetes_order Dothideomycetes_family Eucarya, Fungi Ascomycota Dothideomycetes Hysteriales Hysteriaceae Eucarya, Fungi Ascomycota Dothideomycetes Mytilinidiales Mytilinidiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Dacampiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Leptosphaeriaceae Page 1 of 8 Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Lophiostomataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Massarinaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Melanommataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleomassariaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales_family Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Sporormiaceae Eucarya, Fungi Ascomycota Dothideomycetes -

Particle-Size Distributions and Seasonal Diversity of Allergenic and Pathogenic Fungi in Outdoor Air
The ISME Journal (2012) 6, 1801–1811 & 2012 International Society for Microbial Ecology All rights reserved 1751-7362/12 www.nature.com/ismej ORIGINAL ARTICLE Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air Naomichi Yamamoto1,2, Kyle Bibby1, Jing Qian1,4, Denina Hospodsky1, Hamid Rismani-Yazdi1,5, William W Nazaroff3 and Jordan Peccia1 1Department of Chemical and Environmental Engineering, Yale University, New Haven, CT, USA; 2Japan Society for the Promotion of Science, Tokyo, Japan and 3Department of Civil and Environmental Engineering, University of California, Berkeley, CA, USA Fungi are ubiquitous in outdoor air, and their concentration, aerodynamic diameters and taxonomic composition have potentially important implications for human health. Although exposure to fungal allergens is considered a strong risk factor for asthma prevalence and severity, limitations in tracking fungal diversity in air have thus far prevented a clear understanding of their human pathogenic properties. This study used a cascade impactor for sampling, and quantitative real-time PCR plus 454 pyrosequencing for analysis to investigate seasonal, size-resolved fungal commu- nities in outdoor air in an urban setting in the northeastern United States. From the 20 libraries produced with an average of B800 internal transcribed spacer (ITS) sequences (total 15 326 reads), 12 864 and 11 280 sequences were determined to the genus and species levels, respectively, and 558 different genera and 1172 different species were identified, including allergens and infectious pathogens. These analyses revealed strong relationships between fungal aerodynamic diameters and features of taxonomic compositions. The relative abundance of airborne allergenic fungi ranged from 2.8% to 10.7% of total airborne fungal taxa, peaked in the fall, and increased with increasing aerodynamic diameter. -

Monilinia Species of Fruit Decay: a Comparison Between Biological and Epidemiological Data ______Alessandra Di Francesco, Marta Mari
A. Di Francesco, M. Mari Italian Journal of Mycology vol. 47 (2018) ISSN 2531-7342 DOI: https://doi.org/10.6092/issn.2531-7342/7817 Monilinia species of fruit decay: a comparison between biological and epidemiological data ___________________________________________ Alessandra Di Francesco, Marta Mari CRIOF - Department of Agricultural and Food Science, Alma Mater Studiorum University of Bologna Via Gandolfi, 19, 40057 Cadriano, Bologna, Italy Correspondig Author e-mail: [email protected] Abstract The fungal genus Monilinia Honey includes parasitic species of Rosaceae and Ericaceae. The Monilinia genus shows a great heterogeneity, it is divided in two sections: Junctoriae and Disjunctoriae. These sections were defined by Batra (1991) on the basis of morphology, infection biology, and host specialization. Junctoriae spp. produce mitospore chains without disjunctors, they are parasites of Rosaceae spp.; M. laxa, M. fructicola, M. fructigena and recently M. polystroma, represent the principal species of the section, and they are responsible of economically important diseases of Rosaceae, new species such as M. yunnanensis, and M. mumecola could afflict European fruits in the future in absence of a strict phytosanitary control. The Disjunctoriae section includes species that produce mitospores intercalated by appendages (disjunctors); they parasitize Rosaceae, Ericaceae, and Empetraceae. The principal Disjunctoriae species are M. vaccinii-corymbosi, M. urnula, M. baccarum, M. oxycocci, and M. linhartiana. This study has the aim to underline the importance of Monilinia spp., and to describe their features. Keywords: Monilinia spp; Junctoriae; Disjunctoriae; Prunus; Vaccinium Riassunto Il genere Monilinia Honey include diverse specie che attaccano in particolar modo le piante delle famiglie Rosaceae ed Ericaceae. Monilinia è un genere molto eterogeneo, infatti è suddiviso in due sezioni: Junctoriae e Disjunctoriae.