Vision with Spectacles in Keratoconus Thesis Presented in Partial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Visual Acuity Assessment
VISUAL ACUITY ASSESSMENT J. R. WEATHERILL Bradford Tests of visual function in cataract patients are performed recognised that patients with macular dysfunction have in order to answer two questions: (I) To what extent is the difficulty in reading out of proportion to their Snellen cataract responsible for the patient's symptoms? (2) Will acuity. This difficultyis probably due to parafoveal scoto removal of the cataract improve the patient's sight? To mas which prevent the whole word being seen at the same answer the firstquestion the optical qualities of the eye are time. If a patient reports that the main problem is with assessed, and to answer the second tests are employed reading, it is helpful to observe whether the patient holds which assess retinal and neural function and which are the page steadily or keeps moving it to avoid scotomas. relatively unaffected by the optical degradation caused by With current reading tests patients with poor macular opacities in the media. At Bradford we employ the tests function can read small print for brief periods, particularly listed in Table I. if they are used to reading and recognise the overall shape of the words. A Logmar equivalent for near vision com Snellen Acuity prising random words of equal length and composed of Although the Snellen acuity test measures the function of letters without either ascenders or descenders, read under only the central 1_20 in monochrome at 100% contrast, it controlled conditions of illumination and at a standard dis is nonetheless the 'gold standard' by which ophthal tance, would provide a more accurate measure of macular mologists judge and are judged. -

GUIDE for the Evaluation of VISUAL Impairment
International Society for Low vision Research and Rehabilitation GUIDE for the Evaluation of VISUAL Impairment Published through the Pacific Vision Foundation, San Francisco for presentation at the International Low Vision Conference VISION-99. TABLE of CONTENTS INTRODUCTION 1 PART 1 – OVERVIEW 3 Aspects of Vision Loss 3 Visual Functions 4 Functional Vision 4 Use of Scales 5 Ability Profiles 5 PART 2 – ASSESSMENT OF VISUAL FUNCTIONS 6 Visual Acuity Assessment 6 In the Normal and Near-normal range 6 In the Low Vision range 8 Reading Acuity vs. Letter Chart Acuity 10 Visual Field Assessment 11 Monocular vs. Binocular Fields 12 PART 3 – ESTIMATING FUNCTIONAL VISION 13 A General Ability Scale 13 Visual Acuity Scores, Visual Field Scores 15 Calculation Rules 18 Functional Vision Score, Adjustments 20 Examples 22 PART 4 – DIRECT ASSESSMENT OF FUNCTIONAL VISION 24 Vision-related Activities 24 Creating an Activity Profile 25 Participation 27 PART 5 – DISCUSSION AND BACKGROUND 28 Comparison to AMA scales 28 Statistical Use of the Visual Acuity Score 30 Comparison to ICIDH-2 31 Bibliography 31 © Copyright 1999 by August Colenbrander, M.D. All rights reserved. GUIDE for the Evaluation of VISUAL Impairment Summer 1999 INTRODUCTION OBJECTIVE Measurement Guidelines for Collaborative Studies of the National Eye Institute (NEI), This GUIDE presents a coordinated system for the Bethesda, MD evaluation of the functional aspects of vision. It has been prepared on behalf of the International WORK GROUP Society for Low Vision Research and Rehabilitation (ISLRR) for presentation at The GUIDE was approved by a Work Group VISION-99, the fifth International Low Vision including the following members: conference. -

Contrast Sensitivity and Visual Acuity Among the Elderly
Contrast Sensitivity and Visual Acuity among the Elderly THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Mawada Osman Graduate Program in Vision Science The Ohio State University 2020 Master's Examination Committee: Angela M. Brown, PhD, Advisor Bradley E. Dougherty, OD, PhD Heidi A. Wagner, OD, MPH Copyright by Mawada Osman 2020 Abstract Purpose: To establish the clinical utility and strengthen the validity of the Ohio Contrast Cards (OCC), expand the use of the OCC in a healthy elderly population, and form a baseline dataset of patients to be compared to patients with dementia. Method: Participants ages 65 and over (N = 51) were recruited from the Ohio State University Primary Vision Care (PVC). We assessed the visual function of each patient using four visual tests which include: OCC, Pelli-Robson Chart (PR), Teller Acuity Cards (TAC) and Clear Chart. The contrast sensitivity tests (OCC and PR) were assessed twice, once by each tester. The PR contrast levels were also evaluated at two different distances 1 meter and 3 meters (0.50 meter if visual acuity worse than 6.0 cy/cm). Cognitive abilities were evaluated using the 6-Item Cognitive Impairment Test (6-CIT). Results: A significant effect of test was revealed (p < 0.005), in favor of OCC, yielding consistently higher average LogCS scores than PR, average difference of 0.412 LogCS. The PR and OCC revealed similar repeatability with 95% LoA of ± 0.28 log units and 95% LoA of ± 0.27 log units, respectively. -

Visual Performance of Scleral Lenses and Their Impact on Quality of Life In
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE Visual performance of scleral lenses and their impact on quality of life in patients with irregular corneas Desempenho visual das lentes esclerais e seu impacto na qualidade de vida de pacientes com córneas irregulares Dilay Ozek1, Ozlem Evren Kemer1, Pinar Altiaylik2 1. Department of Ophthalmology, Ankara Numune Education and Research Hospital, Ankara, Turkey. 2. Department of Ophthalmology, Ufuk University Faculty of Medicine, Ankara, Turkey. ABSTRACT | Purpose: We aimed to evaluate the visual quality CCS with scleral contact lenses were 0.97 ± 0.12 (0.30-1.65), 1.16 performance of scleral contact lenses in patients with kerato- ± 0.51 (0.30-1.80), and 1.51 ± 0.25 (0.90-1.80), respectively. conus, pellucid marginal degeneration, and post-keratoplasty Significantly higher contrast sensitivity levels were recorded astigmatism, and their impact on quality of life. Methods: with scleral contact lenses compared with those recorded with We included 40 patients (58 eyes) with keratoconus, pellucid uncorrected contrast sensitivity and spectacle-corrected contrast marginal degeneration, and post-keratoplasty astigmatism who sensitivity (p<0.05). We found the National Eye Institute Visual were examined between October 2014 and June 2017 and Functioning Questionnaire overall score for patients with scleral fitted with scleral contact lenses in this study. Before fitting contact lens treatment to be significantly higher compared with scleral contact lenses, we noted refraction, uncorrected dis- that for patients with uncorrected sight (p<0.05). Conclusion: tance visual acuity, spectacle-corrected distance visual acuity, Scleral contact lenses are an effective alternative visual correction uncorrected contrast sensitivity, and spectacle-corrected contrast method for keratoconus, pellucid marginal degeneration, and sensitivity. -

Higher-Order Wavefront Aberration and Letter-Contrast Sensitivity In
Eye (2008) 22, 1488–1492 & 2008 Macmillan Publishers Limited All rights reserved 0950-222X/08 $32.00 www.nature.com/eye 1 1 2 2 CLINICAL STUDY Higher-order C Okamoto , F Okamoto , T Samejima , K Miyata and T Oshika1 wavefront aberration and letter-contrast sensitivity in keratoconus Abstract Keywords: keratoconus; wavefront aberration; higher-order aberration; contrast sensitivity; Aims To evaluate the relation between letter-contrast sensitivity higher-order aberration of the eye and contrast sensitivity function in eyes with keratoconus. Methods In 22 eyes of 14 patients with Introduction keratoconus (age 30.578.4 years, means7SD) and 26 eyes of 13 normal controls (age Keratoconus is a chronic, non-inflammatory 29.276.7 years), ocular higher-order wavefront disease of the cornea associated with aberration for a 6-mm pupil was measured progressive thinning and anterior protrusion of with the Hartmann-Schack aberrometer the central cornea.1,2 Irregular astigmatism is (KR-9000 PW, Topcon). The root mean square often the first and most apparent clinical finding (RMS) of third- and fourth-order Zernike in keratoconus, and this is evidenced by a coefficients was used to represent higher-order distortion of the corneal image as noted with aberrations. The letter-contrast sensitivity was the placido disc, retinoscope, keratometer, examined using the CSV-1000LV contrast chart keratoscope, and computerized (Vector Vision). videokeratograph. Previous studies using Results In the keratoconus group, the videokeratography have quantitatively 1 Department of letter-contrast sensitivity showed significant demonstrated that corneal irregular Ophthalmology, Institute of correlation with third-order (Spearman’s astigmatism is significantly greater in eyes with Clinical Medicine, University correlation coefficient ¼À0.736, 0.001) 3–6 of Tsukuba, Ibaraki, Japan r Po keratoconus than in normal eyes. -

Fact Sheet: Refractive Errors
Fact Sheet: Refractive Errors More than 11 million Americans have common vision problems that can be corrected with the use of prescriptive eyewear such as glasses or contact lenses.1 These conditions are known as refractive errors and they occur when the eye doesn’t correctly bend, or ―refract,‖ light as it enters the eye. Common refractive errors include the following: o Nearsightedness (also called myopia)—A condition where objects up close appear clearly, while objects far away appear blurry. With nearsightedness, light comes to focus in front of the retina instead of on the retina. o Farsightedness (also called hyperopia)—A common type of refractive error where distant objects may be seen more clearly than objects that are near. However, people experience farsightedness differently. Some people may not notice any problems with their vision, especially when they are young. For people with significant farsightedness, vision can be blurry for objects at any distance, near or far. o Astigmatism—A condition in which the eye does not focus light evenly onto the retina, the light-sensitive tissue at the back of the eye. This can cause images to appear blurry and stretched out. o Presbyopia—An age-related condition in which the ability to focus up close becomes more difficult. As the eye ages, the lens can no longer change shape enough to allow the eye to focus close objects clearly. Refractive errors are one of the most common—and correctable—causes of visual impairment in the United States. According to a recent study led by the National Eye Institute (NEI), approximately half of all American adults don’t have the 20/20 vision physicians consider optimal due to refractive errors.2 Women experience refractive error more frequently than men: Twenty-six percent more women aged 12 and older have uncorrected visual impairment due to refractive error compared with men aged 12 and older. -

Bass – Glaucomatous-Type Field Loss Not Due to Glaucoma
Glaucoma on the Brain! Glaucomatous-Type Yes, we see lots of glaucoma Field Loss Not Due to Not every field that looks like glaucoma is due to glaucoma! Glaucoma If you misdiagnose glaucoma, you could miss other sight-threatening and life-threatening Sherry J. Bass, OD, FAAO disorders SUNY College of Optometry New York, NY Types of Glaucomatous Visual Field Defects Paracentral Defects Nasal Step Defects Arcuate and Bjerrum Defects Altitudinal Defects Peripheral Field Constriction to Tunnel Fields 1 Visual Field Defects in Very Early Glaucoma Paracentral loss Early superior/inferior temporal RNFL and rim loss: short axons Arcuate defects above or below the papillomacular bundle Arcuate field loss in the nasal field close to fixation Superotemporal notch Visual Field Defects in Early Glaucoma Nasal step More widespread RNFL loss and rim loss in the inferior or superior temporal rim tissue : longer axons Loss stops abruptly at the horizontal raphae “Step” pattern 2 Visual Field Defects in Moderate Glaucoma Arcuate scotoma- Bjerrum scotoma Focal notches in the inferior and/or superior rim tissue that reach the edge of the disc Denser field defects Follow an arcuate pattern connected to the blind spot 3 Visual Field Defects in Advanced Glaucoma End-Stage Glaucoma Dense Altitudinal Loss Progressive loss of superior or inferior rim tissue Non-Glaucomatous Etiology of End-Stage Glaucoma Paracentral Field Loss Peripheral constriction Hereditary macular Loss of temporal rim tissue diseases Temporal “islands” Stargardt’s macular due -

Ophthalmology Abbreviations Alphabetical
COMMON OPHTHALMOLOGY ABBREVIATIONS Listed as one of America’s Illinois Eye and Ear Infi rmary Best Hospitals for Ophthalmology UIC Department of Ophthalmology & Visual Sciences by U.S.News & World Report Commonly Used Ophthalmology Abbreviations Alphabetical A POCKET GUIDE FOR RESIDENTS Compiled by: Bryan Kim, MD COMMON OPHTHALMOLOGY ABBREVIATIONS A/C or AC anterior chamber Anterior chamber Dilators (red top); A1% atropine 1% education The Department of Ophthalmology accepts six residents Drops/Meds to its program each year, making it one of nation’s largest programs. We are anterior cortical changes/ ACC Lens: Diagnoses/findings also one of the most competitive with well over 600 applicants annually, of cataract whom 84 are granted interviews. Our selection standards are among the Glaucoma: Diagnoses/ highest. Our incoming residents graduated from prestigious medical schools ACG angle closure glaucoma including Brown, Northwestern, MIT, Cornell, University of Michigan, and findings University of Southern California. GPA’s are typically 4.0 and board scores anterior chamber intraocular ACIOL Lens are rarely lower than the 95th percentile. Most applicants have research lens experience. In recent years our residents have gone on to prestigious fellowships at UC Davis, University of Chicago, Northwestern, University amount of plus reading of Iowa, Oregon Health Sciences University, Bascom Palmer, Duke, UCSF, Add power (for bifocal/progres- Refraction Emory, Wilmer Eye Institute, and UCLA. Our tradition of excellence in sives) ophthalmologic education is reflected in the leadership positions held by anterior ischemic optic Nerve/Neuro: Diagno- AION our alumni, who serve as chairs of ophthalmology departments, the dean neuropathy ses/findings of a leading medical school, and the director of the National Eye Institute. -

Visual Optics
Visual Optics Jim Schwiegerling, PhD Ophthalmology & Optical Sciences University of Arizona Visual Optics - Introduction • In this course, the optical principals behind the workings of the eye and visual system will be explained. • Furthermore, descriptions of different devices used to measure the properties and functioning of the eye will be described. • Goals are a general understanding of how the visual system works and exposure to an array of different optical instrumentation. 1 What is Vision? • Three pieces are needed for a visual system – Eye needs to form an “Image”. Image is loosely defined because it can be severely degraded and still provide information. – Image needs to be converted to a neural signal and sent to the brain. – Brain needs to interpret and process the image. Analogous Vision System Feedback Video Computer Camera Analysis Display Conversion & Image To Signal Interpretation Formation 2 Engineering Analysis • Optics – Aberrations and raytracing are used to determine the optical properties of the eye. Note that the eye is non- rotationally symmetric, so analysis is more complex than systems normally discussed in lens design. Fourier theory can also be used to determine retinal image with the point spread function and optical transfer function. •Neural– Sampling occurs by the photoreceptors (both spatial and quantification), noise reduction, edge filtering, color separation and image compression all occur in this stage. •Brain– Motion analysis, pattern recognition, object recognition and other processing occur in the brain. Anatomy of the Eye Anterior Chamber –front portion of the eye Cornea –Clear membrane on the front of the eye. Aqueous – water-like fluid behind cornea. Iris – colored diaphragm that acts as the aperture stop of the eye. -

Contrast Sensitivity and Visual Acuity in Low-Vision Students Thesis
Contrast Sensitivity and Visual Acuity in Low-Vision Students Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Steve Murimi Mathenge Njeru, BS Graduate Program in Vision Science The Ohio State University 2020 Thesis Committee Angela M. Brown, PhD, Advisor Bradley E. Dougherty, OD, PhD Deyue Yu, PhD Copyrighted by Steve Murimi Mathenge Njeru 2020 Abstract Purpose: This study primarily compared the test-retest reliability of the Pelli-Robson chart (PR) and Ohio Contrast Cards (OCC) amongst testers. The secondary goal of this study was to examine the impact on contrast sensitivity if the testing distance for the Pelli-Robson chart were to be changed. An additional goal was to evaluate the relationship between visual acuity (VA) and contrast sensitivity (CS) when using letter-based charts and grating cards. Methods: Thirty low-vision students were tested, ranging from 7-20 years old. Each student was tested with both VA and CS tests in randomized order, which included: the Bailey- Lovie chart (BL), Pelli-Robson chart, Teller Acuity Cards (TAC), and Ohio Contrast Cards. Each student repeated both the PR chart and OCC in separate rooms, but neither the BL chart nor TAC was repeated. The PR chart was also tested at closer testing distance, based on the student’s logMAR acuity from the BL chart. For the letter charts, a letter-by-letter scoring method was used. For grating cards, these were both scored as preferential looking tests. Results: The Limits of Agreement for the OCC and PR chart were +/- 0.451 and +/- 0.536, respectively. -

Scleral Lenses in the Management of Keratoconus
ARTICLE Scleral Lenses in the Management of Keratoconus Muriel M. Schornack, O.D., and Sanjay V. Patel, M.D. Management of patients with keratoconus consists primarily of Purpose: To describe the use of Jupiter scleral lenses (Medlens Innova- providing optical correction to maximize visual function. In very tions, Front Royal, VA; and Essilor Contact Lenses, Inc., Dallas, TX) in the management of keratoconus. mild or early disease, spectacle correction or standard hydrogel or Methods: We performed a single-center retrospective chart review of our silicone hydrogel lenses may provide adequate vision. However, initial 32 patients with keratoconus evaluated for scleral lens wear. All disease progression results in increasing ectasia, which gives rise patients were referred for scleral lens evaluation after exhausting other to complex optical aberrations. Rigid gas-permeable contact lenses nonsurgical options for visual correction. Diagnostic lenses were used in mask these aberrations by allowing a tear lens to form between the the initial fitting process. If adequate fit could not be achieved with contact lens and the irregular corneal surface. Zadnik et al. found standard lenses, custom lenses were designed in consultation with the that 65% of patients who enrolled in the Collaborative Longitudi- manufacturers’ specialists. The following measures were evaluated for nal Evaluation of Keratoconus study were wearing rigid gas- each patient: ability to tolerate and handle lenses, visual acuity with scleral permeable lenses in one or both eyes at the time of enrollment.4 lenses, number of lenses, and visits needed to complete the fitting process. Despite their optical benefit, corneal rigid gas-permeable lenses Results: Fifty-two eyes of 32 patients were evaluated for scleral lens may not be appropriate for all patients with keratoconus. -
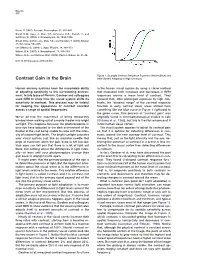
Contrast Gain in the Brain After (Green) Adapting to High Contrasts
Neuron 476 Rozin, P. (1982). Percept. Psychophys. 31, 397–401. Small, D.M., Voss, J., Mak, Y.E., Simmons, K.B., Parrish, T., and Gitelman, D. (2004). J. Neurophysiol. 92, 1892–1903. Small, D.M., Gerber, J.C., Mak, Y.E., and Hummel, T. (2005). Neuron 47, this issue, 593–605. von Békésy, G. (1964). J. Appl. Physiol. 19, 369–373. Wilson, D.A. (1997). J. Neurophysiol. 78, 160–169. Wilson, D.A., and Sullivan, R.M. (1999). Physiol. Behav. 66, 41–44. DOI 10.1016/j.neuron.2005.08.002 Figure 1. Example Contrast Response Functions Before (Blue) and Contrast Gain in the Brain After (Green) Adapting to High Contrasts Human sensory systems have the remarkable ability in the human visual system by using a clever method of adjusting sensitivity to the surrounding environ- that measured both increases and decreases in fMRI ment. In this issue of Neuron, Gardner and colleagues responses around a mean level of contrast. They used fMRI to show how the visual system shifts its showed that, after prolonged exposure to high con- sensitivity to contrast. This process may be helpful trasts, the “dynamic range” of the contrast response for keeping the appearance of contrast constant function in early cortical visual areas shifted from across a range of spatial frequencies. something like the blue curve in Figure 1 rightward to the green curve. This process of “contrast gain” was We’ve all had the experience of being temporarily originally found in electrophysiological studies in cats blinded when walking out of a movie theater into bright (Ohzawa et al., 1985), but this is the first evidence of it sunlight.