Thyroid and Adrenal Relationships VICTOR PARSONS IAN RAMSAY D.M., M.R.C.P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Procedure Manual
Laboratory Procedure Manual Analyte: Thyroid Stimulating Hormone Matrix: Serum Method: Microparticle Enzyme Immunoassay (MEIA) Method No.: Revised: as performed by: Coulston Foundation Alamogordo, New Mexico Contact: Ms. Love Julian 1-505-434-1725 Important Information for Users Coulston periodically refines these laboratory methods. It is the responsibility of the user to contact the person listed on the title page of each write-up before using the analytical method to find out whether any changes have been made and what revisions, if any, have been incorporated. Thyroid Stimulating Hormone NHANES 2001–2002 Public Release Data Set Information This document details the Lab Protocol for NHANES 2001-2002 data. Two laboratories performed this testing during 2001-2002. In order to maintain confidentiality of the participants the quality control summary statistics and graphs were combined to mask the individual analysis dates from the two laboratories. Methods for both labs are included in this release. The method for Lab18 analyte is included in this file. The method for Lab40 is described in a separate file. A tabular list of the released analytes follows: Lab Number Analyte SAS Label lab18 LBXTSH Thyroid Stimulating Hormone Page 2 of 11 Thyroid Stimulating Hormone NHANES 2001-2002 1. Summary of Test Principle and Clinical Relevance IMx Ultrasensitive hTSH II is a Microparticle Enzyme Immunoassay (MEIA) for the quantitative determination of human thyroid stimulating hormone (hTSH) in serum or plasma on the IMx analyzer. The IMx Ultrasensitive hTSH II assay is based on the MEIA technology. The IMx Ultrasensitive hTSH II reagents and sample are added to the reaction cell in the following sequence: The probe/electrode assembly delivers the sample and anti-hTSH coated microparticles to the incubation well of the reaction cell. -

Congenital Adrenal Hyperplasia in the Newborn
The Leo Fung Center for CAH and Disorders of Sex Development Congenital Adrenal Hyperplasia in the Newborn Contents Introduction 1 What is congenital adrenal hyperplasia? 1 Types of CAH 3 Diagnosing CAH in newborns 4 Treating CAH 5 Untreated CAH 7 CAH in children and young adults 8 Frequently asked questions 9 Glossary 11 Resources 13 Acknowledgments 14 Congenital Adrenal Hyperplasia in the Newborn 1 Introduction This handbook will provide you and your family information about congenital adrenal hyperplasia (CAH). While this guide will not answer all of your questions, it will provide basic medical facts that will help you to talk to your doctors. It is important to know that CAH cannot be cured but it can be treated. Your child will need to take medicine for the rest of his or her life. If your child takes this medicine, he or she should have a completely normal life in every way. Successful treatment requires teamwork between you and your doctor. The doctor will monitor your child in order to know what dose of medicine is needed. We ask that you give your baby the medication on the schedule recommended by your doctor. Your family is not alone. The Leo Fung Center for CAH and Disorders of Sex Development (DSD) at University of Minnesota Amplatz Children’s Hospital, provides a large network of support, including medical specialists, therapists and counselors who all have expertise in caring for patients with CAH. What is congenital adrenal hyperplasia? Let’s begin by examining each word. • Congenital means existing at birth (inherited). • Adrenal means that the adrenal glands are involved. -

Conduct Protocol in Emergency: Acute Adrenal Insufficiency
ORIGINAL ARTICLE FARES AND SANTOS Conduct protocol in emergency: Acute adrenal insufficiency ADIL BACHIR FARES1*, RÔMULO AUGUSTO DOS SANTOS2 1Medical Student, 6th year, Faculdade de Medicina de São José do Rio Preto (Famerp), São José do Rio Preto, SP, Brazil 2Degree in Endocrinology and Metabology from Sociedade Brasileira de Endocrinologia e Metabologia (SBEM). Assistant Physician at the Internal Medicine Service of Hospital de Base. Researcher at Centro Integrado de Pesquisa (CIP), Hospital de Base, São José do Rio Preto. Endocrinology Coordinator of the Specialties Outpatient Clinic (AME), São José do Rio Preto, SP, Brazil SUMMARY Introduction: Acute adrenal insufficiency or addisonian crisis is a rare comor- bidity in emergency; however, if not properly diagnosed and treated, it may progress unfavorably. Objective: To alert all health professionals about the diagnosis and correct treatment of this complication. Method: We performed an extensive search of the medical literature using spe- cific search tools, retrieving 20 articles on the topic. Results: Addisonian crisis is a difficult diagnosis due to the unspecificity of its signs and symptoms. Nevertheless, it can be suspected in patients who enter the emergency room with complaints of abdominal pain, hypotension unresponsive to volume or vasopressor agents, clouding, and torpor. This situation may be associated with symptoms suggestive of chronic adrenal insufficiency such as hyperpigmentation, salt craving, and association with autoimmune diseases such as vitiligo and Hashimoto’s thyroiditis. Hemodynamically stable patients Study conducted at Faculdade may undergo more accurate diagnostic methods to confirm or rule out addiso- de Medicina de São José do nian crisis. Delay to perform diagnostic tests should be avoided, in any circum- Rio Preto (Famerp), São José do Rio Preto, SP, Brazil stances, and unstable patients should be immediately medicated with intravenous glucocorticoid, even before confirmatory tests. -

Preventable Deaths: Panhypopituitarism and Adrenal Insufficiency
Preventable Deaths: Panhypopituitarism and adrenal insufficiency. What you need to know What is panhypopituitarism? Your child has been diagnosed with a big scary sounding word, and all you can think is: 'What does this mean? and 'Why have I never heard of this?' Simply put, panhypopituitarism means that your child's pituitary gland does not function properly and as a result, your child is deficient in one or several hormones. Some children have congenital panhypopituitarism, meaning they are born with it. Others have acquired panhypopituitarism following an event such as head trauma, brain tumor surgery, or brain radiation. It is rare enough that is entirely possible, in fact, probable, that you will not initially know anyone else with this disorder. What will my child need? Although the diagnosis and condition can seem intimidating, it is very manageable once you understand what is needed. Unfortunately the condition cannot be cured or reversed, but again, it can be effectively managed. Your child will need to take medications to replace the missing hormones. These might include thyroid hormone, growth hormone, cortisol, and/or possibly others. Your doctor will go over these with you, as dosages vary from child to child. Most medications are taken orally, but growth hormone must be taken by daily injection. Your endocrinologist will work with you and your child to achieve the proper dosages and will guide you in how to administer any necessary medications. Why is it sometimes life threatening? What is adrenal insufficiency? Of the hormone deficiencies your child may have, the most critical is cortisol, also known as the 'stress hormone.' Cortisol is essential for life , and is therefore the central focus of this guide. -

Thyroid Follicular Adenoma: Benign Or Malignant?
Volume 4 Number 3 Medical Journal of the ['ayiz1369 Islamic Repuhlit of Imn Rabiolawwal141 I F:llll990 THYROID FOLLICULAR ADENOMA: BENIGN OR MALIGNANT? HOSSEIN GHARIB, M.D. From fhe Division of Endocrillology and !lIlernal Medicine, Mayo Clinic and Mayo FOll1uiafion, Rochester, MillllCSOla, U.S.A. ABSTRACT Four patients are described in whom a follicular carcinoma developed following thyroidectomy for a benign follicular neoplasm. It is possible that the initial thyroid neoplasm was a well- differentiated follicular carcinoma which was microscopically indistinguishable from a benign adenoma. Realizing this pathologic pitfall in thyroid diagnosis, the need for meticulous examination of the pathologic specimen is emphasized. Long- term postop erative reassessment is recommended. MIIRI, Vol. 4, No.3, 173-176, 1990 INTRODUCTION CASE REPORTS Follicular adenoma is the most common type of Case I cellular thyroid adenomas. 1 There is considerable de A 49-year-old woman was referred for evaluation of bate whether follicular adenoma of the thyroid, a metastatic thyroid carcinoma. She was in good health benign neoplasm, is a precancerous lesion which occa until six months earlier when she complained of ins om- sionally may be mistaken for a carcinoma2.30n the nia and nervousness. Two months before admission a other hand, several published reports indicate that a routine chest x-ray revealed metastatic nodules in both follicular adenoma which appears benign by conven lungs. Extensive laboratory tests and radiographic tional histologic criteria, may demonstrate malignant studies were negative. A diagnostic left thoracotomy behavior."·() showed ,dow grade thyroid cancep. and she was refer This report describes four patients whose thyroid red for further examination. -
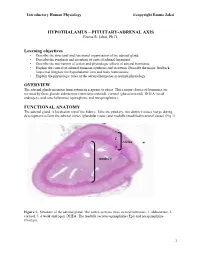
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

Comprehensive Assessment of TERT Mrna Expression Across a Large Cohort of Benign and Malignant Thyroid Tumours
cancers Article Comprehensive Assessment of TERT mRNA Expression across a Large Cohort of Benign and Malignant Thyroid Tumours Ana Pestana 1,2,3, Rui Batista 1,2,3, Ricardo Celestino 1,2,3,4 , Sule Canberk 1,2,5, Manuel Sobrinho-Simões 1,2,3,6 and Paula Soares 1,2,3,7,* 1 Institute of Molecular Pathology and Immunology of the University of Porto (Ipatimup), 4200-135 Porto, Portugal; [email protected] (A.P.); [email protected] (R.B.); [email protected] (R.C.); [email protected] (S.C.); [email protected] (M.S.-S.) 2 i3S-Instituto de Investigação e Inovação em Saúde, Universidade do Porto, 4200-135 Porto, Portugal 3 Medical Faculty of University of Porto (FMUP), 4200-139 Porto, Portugal 4 School of Allied Health Technologies, Polytechnic of Porto, 4200-072 Porto, Portugal 5 Abel Salazar Biomedical Sciences Institute (ICBAS), University of Porto, 4050-313 Porto, Portugal 6 Department of Pathology, Centro Hospitalar São João, 4200-139 Porto, Portugal 7 Department of Pathology, Medical Faculty of the University of Porto, 4200-139 Porto, Portugal * Correspondence: [email protected]; Tel.: +351-220-408-800 Received: 10 June 2020; Accepted: 6 July 2020; Published: 9 July 2020 Abstract: The presence of TERT promoter (TERTp) mutations in thyroid cancer have been associated with worse prognosis features, whereas the extent and meaning of the expression and activation of TERT in thyroid tumours is still largely unknown. We analysed frozen samples from a series of benign and malignant thyroid tumours, displaying non-aggressive features and low mutational burden in order to evaluate the presence of TERTp mutations and TERT mRNA expression in these settings. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Thyroid Hormones in Fetal Growth and Prepartum Maturation
A J FORHEAD and A L FOWDEN Thyroid hormones and fetal 221:3 R87–R103 Review development Thyroid hormones in fetal growth and prepartum maturation A J Forhead1,2 and A L Fowden1 Correspondence should be addressed 1Department of Physiology, Development and Neuroscience, University of Cambridge, Physiology Building, to A L Fowden Downing Street, Cambridge CB2 3EG, UK Email 2Department of Biological and Medical Sciences, Oxford Brookes University, Oxford OX3 0BP, UK [email protected] Abstract The thyroid hormones, thyroxine (T4) and triiodothyronine (T3), are essential for normal Key Words growth and development of the fetus. Their bioavailability in utero depends on " thyroid hormones development of the fetal hypothalamic–pituitary–thyroid gland axis and the abundance " intrauterine growth of thyroid hormone transporters and deiodinases that influence tissue levels of bioactive " maturation hormone. Fetal T4 and T3 concentrations are also affected by gestational age, nutritional and " neonatal adaptation endocrine conditions in utero, and placental permeability to maternal thyroid hormones, which varies among species with placental morphology. Thyroid hormones are required for the general accretion of fetal mass and to trigger discrete developmental events in the fetal brain and somatic tissues from early in gestation. They also promote terminal differentiation of fetal tissues closer to term and are important in mediating the prepartum maturational effects of the glucocorticoids that ensure neonatal viability. Thyroid hormones act directly through anabolic effects on fetal metabolism and the stimulation of fetal oxygen Journal of Endocrinology consumption. They also act indirectly by controlling the bioavailability and effectiveness of other hormones and growth factors that influence fetal development such as the catecholamines and insulin-like growth factors (IGFs). -

Beyond the Fixed Setpoint of the Hypothalamus–Pituitary–Thyroid Axis
E Fliers and others Hypothalamus–pituitary– 171:5 R197–R208 Review thyroid axis MECHANISMS IN ENDOCRINOLOGY Beyond the fixed setpoint of the hypothalamus–pituitary–thyroid axis Eric Fliers1, Andries Kalsbeek1,2 and Anita Boelen1 Correspondence should be addressed 1Department of Endocrinology and Metabolism, Academic Medical Center, University of Amsterdam, 1105 AZ to E Fliers Amsterdam, The Netherlands and 2Hypothalamic Integration Mechanisms, Netherlands Institute for Neuroscience, Email Amsterdam, The Netherlands e.fl[email protected] Abstract The hypothalamus–pituitary–thyroid (HPT) axis represents a classical example of an endocrine feedback loop. This review discusses dynamic changes in HPT axis setpoint regulation, identifying their molecular and cellular determinants, and speculates about their functional role. Hypothalamic thyrotropin-releasing hormone neurons were identified as key components of thyroid hormone (TH) setpoint regulation already in the 1980s, and this was followed by the demonstration of a pivotal role for the thyroid hormone receptor beta in negative feedback of TH on the hypothalamic and pituitary level. Gradually, the concept emerged of the HPT axis setpoint as a fixed entity, aiming at a particular TH serum concentration. However, TH serum concentrations appear to be variable and highly responsive to physiological and pathophysiological environmental factors, including the availability or absence of food, inflammation and clock time. During food deprivation and inflammation, TH serum concentrations decrease without a concomitant rise in serum TSH, reflecting a deviation from negative feedback regulation in the HPT axis. Surprisingly, TH action in peripheral organs in these conditions cannot be simply predicted by decreased serum TH concentrations. Instead, diverse environmental stimuli have differential effects on local TH metabolism, e.g. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Using T3 for Treatment of Hypothyroidism - What the Evidence Say?
Journal of Endocrinology and Thyroid Research ISSN: 2573-2188 Review Article J Endocrinol Thyroid Res Volume 2 Issue 2- June 2017 Copyright © All rights are reserved by Vismay Naik, DOI: 10.19080/JETR.2017.02.555584 Using T3 For Treatment of Hypothyroidism - What the Evidence Say? Vismay Naik* Ashirvad Heart and Diabetes Centre, India Submission: March 01, 2017; Published: June 27, 2017 *Corresponding author: Vismay Naik, MD, MRCP (London), Ashirvad Heart and Diabetes Centre, Botad, Gujarat, India, Email: Introduction Hypothyroidism is due to the primary disease of the thyroid Treatment Strategies of Hypothyroidism or secondary to hypothalamic- pituitary disease [1]. Primary The objectives of management of hypothyroidism are to hypothyroidism is the most common endocrine disorder reverse clinical progression, correct metabolic abnormalities, throughout the world. Traditionally we have been using [3] and reduction of goiter size in patients with autoimmune levothyroxine in the treatment of hypothyroidism. However, there Hashimoto’s thyroiditis [4]. This can be achieved by 3 different have been reports of persisting symptoms of hypothyroidism strategies: in 5-10% of levothyroxine treated hypothyroid patients a. Standard treatment with a daily dose levothyroxine (T4) in order to reach an age adjusted TSH target [5]. The with normal serum thyrotrophin (TSH) [2]. Physiologically dose has to be titrated periodically [3]. levothyronine is the active form, so treating the patient with the active form has been considered optimum treatment. This study b. Triiodothyronine (T3) monotherapy is the biological is aimed at reviewing the literature on using T3 in treatment of hypothyroidism either alone or in combination with T4. By the its short half-life [6] end of this study we hope to answer the question, if the use of T3 active form.