N92-12929 54Th MET SOC 231
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Geological Newsletter
JAN 90 THE GEOLOGICAL NEWSLETTER ·• GEOLOGICAL SOCIETY OF THE OREGON COUNTRY GEOLOGICAL SOCIETY Non-Profit Org. U.S. POSTAGE OF THE OREGON COUNTRY PAID P.O. BOX ?a 7- Portland, Oregon PORTLAND, OR 97207- -:· ·--~··, Permit No. 999 - -- '~ Dr. Frank Boersma 120 W. 33~d Street Vancouver, WA 98660 GEOLOGICAL SOCIETY OF THE OREGOt\ COllNTRY 1989-1990 ADMINISTRATION BOARD OF DIRECTORS President Directors Rosemary Kenney 221-0757 Peter E. Baer (3 years) 661-7995 4211 S\-1 Condor Charlene Holzwarth (2 years) 284-3444 Portland, OR 97201 Esther Kennedy (1 year) 287-3091 Vice President Margaret L. Steere 246-1670 Immediate Past Presidents Joline Robustelli 223-2852 6929 SW 34 Ave. ~ Portland, OR 97219 R.E. (Andy) Corcoran 244-5605 Secretary Alta B. Fosback 641-6323 THE GEOLOGICAL NEWSLETTER 8942 SW Fairview Place Tigard, OR 97223 Editor: Sandra Anderson 775-5538 Treasurer Calendar: Margaret Steere 246-1670 Braden Pillow 659-6318 Business Manager: Carol Cole 220-0078 19562 SE Cottonwood St. Assist: Cecelia Crater 235-5158 Milwaukie, OR 97267 ACTIVITIES CHAIRS Calligrapher Properties and PA System Wallace R.· McClung 637-3834 (Luncheon) Donald Botteron 245-6251 Field Trips (Evening) Walter A. Sunderland 625-6840 Charlene Holzwarth 284-3444 Publications Alta B. Fosback 641-6323 Geneva E. Reddekopp 654-9818 Geology Seminars Publicity Donald D. Barr 246-2785 Roberta L. Walter 235-3579 Historian Refreshments Phyllis G. Bonebrake 289-8597 (Friday Evening) Hospitality David and Marvel Gillespie 246-2368 254-0135 (Luncheon) Margaret Fink 289-0188 Harold and Patricia Gay Moore (Evening) Maxine Harrington 297-ll86 (Geology Seminars) Catherine Evenson 654-2636 Library: Esther Kennedy 287-3091 ' ' Betty Turner 246-3192 Telephone n Past Presidents Panel Jean L. -

Lafayette - 800 Grams Nakhlite
Lafayette - 800 grams Nakhlite Figure 1. Photograph showing fine ablation features Figure 2. Photograph of bottom surface of Lafayette of fusion crust on Lafayette meteorite. Sample is meteorite. Photograph from Field Museum Natural shaped like a truncated cone. This is a view of the top History, Chicago, number 62918. of the cone. Sample is 4-5 centimeters across. Photo- graph from Field Museum Natural History, Chicago, number 62913. Introduction According to Graham et al. (1985), “a mass of about 800 grams was noticed by Farrington in 1931 in the geological collections in Purdue University in Lafayette Indiana.” It was first described by Nininger (1935) and Mason (1962). Lafayette is very similar to the Nakhla and Governador Valadares meteorites, but apparently distinct from them (Berkley et al. 1980). Lafayette is a single stone with a fusion crust showing Figure 3. Side view of Lafayette. Photograph from well-developed flow features from ablation in the Field Museum Natural History, Chicago, number Earth’s atmosphere (figures 1,2,3). The specimen is 62917. shaped like a rounded cone with a blunt bottom end. It was apparently oriented during entry into the Earth’s that the water released during stepwise heating of atmosphere. Note that the fine ablation features seen Lafayette was enriched in deuterium. The alteration on Lafayette have not been reported on any of the assemblages in Lafayette continue to be an active field Nakhla specimens. of research, because it has been shown that the alteration in Lafayette occurred on Mars. Karlsson et al. (1992) found that Lafayette contained the most extra-terrestrial water of any Martian Lafayette is 1.32 b.y. -

Expedition 330 Core Descriptions, Site U1374 Thin Sections
Site U1374 thin sections U1374A-3R-1-W 114_116 Proc. IODP THIN SECTION: 330-U1374A-3R-1-W 114_116-BILLET 114-SLIDE 114 Piece No: Unit:I OBSERVER:THIN SECTION:SLIDE 114 Site U1374 core descriptions ROCK NAME: moderately olivine-phyric basalt clast WHERE SAMPLED: clast type 2 GRAINSIZE: fine grained TEXTURE: moderately phyric | Volume 330 PRIMARY PERCENT REL. VOL. SIZE(mm) VESICLE VESICLE MINERALOGY ORIGINAL REPLACED min. max. mode. MORPHOLOGY SPHERICITY Infilling [%] COMMENTS PHENOCRYSTS 10 olivine 5 20 8 1.2 skeletal incomplete dome face (iddingsite); "skeletal olivine" & "euhedral to subhedral" MICROPHENOCRYST plagioclase 0.01 0.3 0.4 laths[330] just 2 grains olivine 4 40 0.9 0.4 skeletal incomplete dome face (iddingsite); "skeletal olivine" & "euhedral to subhedral" augite 1 0.3 0.3 subhedral sector-zoning titanaugite VESICLES 3 0.1 1.8 0.6 low[330] 100 filled by calcite GROUNDMASS 87 opaque mineral 5 olivine 4 95 0.09 0.06 subhedral to replaced by iddingsite (hematite?) anhedral[330] glass 68 100 palagonite augite 1 anhedral plagioclase 9 0.2 0.01 microlite[330] SECONDARY SIZE(mm) MINERALOGY min. max. mode. REPLACING/FILLING COMMENTS calcite vesicle STRUCTURE no structure in groundmass COMMENTS SUMMARY DESCRIPTION Thin sections 1 U1374A-3R-2-W 7_9 Proc. IODP THIN SECTION: 330-U1374A-3R-2-W 7_9-BILLET 115-SLIDE 115 Piece No: Unit:I OBSERVER:THIN SECTION:SLIDE 115 Site U1374 core descriptions ROCK NAME: highly olivine-phyric basalt clast WHERE SAMPLED: CLAST TYPE 1 GRAINSIZE: fine grained TEXTURE: highly phyric & glomeroporphyritic | Volume 330 PRIMARY PERCENT REL. -

Aqueous Alteration of Yamato 000749 Based on Multi-Probe Microscopic Observation
Aqueous alteration of Yamato 000749 based on multi-probe microscopic observation Naoki Shiraishi1, Hiroki Suga1,2, Masaaki Miyahara1, Takuji Ohigashi3, Yuichi Inagaki3, Akira Yamaguchi4, Naotaka Tomioka 5, Yu Kodama6, and Eiji Ohtani7 1Hiroshima University, 2The University of Tokyo, 3UVSOR synchrotron facility, 4NIPR, 5Kochi Institute for Core Sample 6 7 Research, JAMSTEC, Marine Works Japan, Tohoku University Motivation Based on the results of Mars explorations and researches of Martian meteorites for many years, there is a high possibility that liquid water had existed extensively on Mars for a long time (Sekine, 2012). Martian meteorite is one of the important clues for restoring the past Martian surface environment that cannot be unveiled by Mars surface survey alone. It is expected that a member of Martian meteorites, nakhlites have evidences for a rock-fluid reaction occurred on Mars. One of the representative evidence for the rock-fluid reaction is “iddingsite”, which is an alteration texture formed in and around olivine grains in nakhlites. Many kinds of secondary minerals occur in the iddingsite through the rock-fluid reaction. The mineral assemblages, chemical compositions and chemical species of the secondary minerals depend on the varied parameters such as temperature and pH of the fluid. Suga et al. (2017) described the secondary minerals in the iddingsite of nakhlites Yamato (Y) 000593. Another Martian meteorites, Yamato (Y) 000749 is also a member of Yamato 00 nakhlites like Y 000593, containing abundant iddingsite. Based on Cohen et al. (2017), Y 000749 was located at the lower portion of the same nakhlites source compared to Y 000593. It is likely that these nakhlites have different mode of alteration even in the same source. -

Alkalic Rocks of Iron Hill Gunnison County, Colorado
If yon do not need this publication after it has served your purpose, please return it to the Geological Survey, using the official mailing label at the end UNITED STATES DEPARTMENT OF THE INTERIOR ALKALIC ROCKS OF IRON HILL GUNNISON COUNTY, COLORADO GEOLOGICAL SURVEY PROFESSIONAL PAPER 197-A UNITED STATES DEPARTMENT OF THE INTERIOR Harold L. Ickes, Secretary GEOLOGICAL SURVEY W. C. Mendenhall, Director Professional Paper 197-A ALKALIC ROCKS OF IRON HILL GUNNISON COUNTY, COLORADO BY ESPER S. LARSEN Shorter contributions to general geology, 1941 (Pages 1-64) UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1942 For sale by the Superintendent of Documents, Washington, D. C. ......... Price 40 cents CONTENTS Page Preface, by G. F. Loughlin_________________________ v Other dike rocks_______________-____________________ 29 Abstract____"_--__-___--__________________________ 1 Augite syenite and shonkinite ____.__--___--__--__ 29 Introduction. ______________________________________ 1 Olivine gabbro. ________________________________ 30 Location and topography. _______________________ 1 Carbonate veins_________----__---------__--_---___- 31 Field work and acknowledgments.________________ 2 Character __ __________________________________ 31 Geology of the surrounding area__________________ 2 Origin _______ _ ____ _ __ _.. __ __. ___ .__. 31 Age of the Iron Hill stock_____________________ 2 Hydrothermal processes- ______--_-___-_---_--__-___- 31 General geology of the Iron Hill stock _____________ 3 The hydrothermal products. _____________________ -
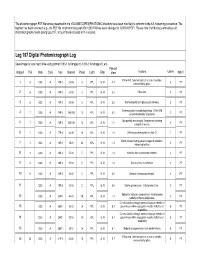
Leg 197 Digital Photomicrograph Log
The photomicrograph PDF filenames presented in the VOLUME\CORES\PHOTOMIC directory have been modified to conform to the 8.3 filenaming convention. The hyphen has been removed (e.g., the PDF file for photomicrograph ID# 1203-100 has been changed to 1203100.PDF). Please note that this log summarizes all photomicrographs taken during Leg 197, not just those included in this volume. Leg 197 Digital Photomicrograph Log Save image to local hard drive using format 1203-1 for image #1, 1203-2 for image #2, etc. Field of Image# TS# Hole Core Sec Interval Piece Light Filter View Feature Unit # Who? Pillow rind. See microlytic pl in core of varioles, 6 1203 A 19R-2 24-26 3 PPL B, N 1.4 3PT 1 surrounded by glass. 2 6 1203 A 19R-2 24-26 3 PPL B, N 5.5 Pillow rind. 3PT 3 6 1203 A 19R-2 24-26 3 PPL B, N 5.5 Glomerocrystic pl in glasssy groundmass. 3PT Glomerocrystic rounded plagioclase. Ol on LHS 4 7 1203 A 19R-2 136-138 15 XPL B, N 5.5 3PT psuedomorphed by magnesite. Cpx partially enclosing pl. Groundmass showing 5 7 1203 A 19R-2 136-138 15 XPL B, N 1.4 3PT subophitic texture. 6 1 1203 A 17R-4 34-38 10 XPL B, N 1.4 Olivine pseudomorphed by silica (?) 1PT Relict olivine showing several stages of alteration. 7 4 1203 A 19R-1 19-21 2A XPL B, N 1.4 3PT Interesting texture. 8 2 1203 A 18R-2 27-30 5 PPL B, N 1.4 Variolitic clast in carbonate interbed. -

The Nakhlite Meteorites: Augite-Rich Igneous Rocks from Mars ARTICLE
ARTICLE IN PRESS Chemie der Erde 65 (2005) 203–270 www.elsevier.de/chemer INVITED REVIEW The nakhlite meteorites: Augite-rich igneous rocks from Mars Allan H. Treiman Lunar and Planetary Institute, 3600 Bay Area Boulevard, Houston, TX 77058-1113, USA Received 22 October 2004; accepted 18 January 2005 Abstract The seven nakhlite meteorites are augite-rich igneous rocks that formed in flows or shallow intrusions of basaltic magma on Mars. They consist of euhedral to subhedral crystals of augite and olivine (to 1 cm long) in fine-grained mesostases. The augite crystals have homogeneous cores of Mg0 ¼ 63% and rims that are normally zoned to iron enrichment. The core–rim zoning is cut by iron-enriched zones along fractures and is replaced locally by ferroan low-Ca pyroxene. The core compositions of the olivines vary inversely with the steepness of their rim zoning – sharp rim zoning goes with the most magnesian cores (Mg0 ¼ 42%), homogeneous olivines are the most ferroan. The olivine and augite crystals contain multiphase inclusions representing trapped magma. Among the olivine and augite crystals is mesostasis, composed principally of plagioclase and/or glass, with euhedra of titanomagnetite and many minor minerals. Olivine and mesostasis glass are partially replaced by veinlets and patches of iddingsite, a mixture of smectite clays, iron oxy-hydroxides and carbonate minerals. In the mesostasis are rare patches of a salt alteration assemblage: halite, siderite, and anhydrite/ gypsum. The nakhlites are little shocked, but have been affected chemically and biologically by their residence on Earth. Differences among the chemical compositions of the nakhlites can be ascribed mostly to different proportions of augite, olivine, and mesostasis. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

OFR 2004-7, a Self-Guided Tour of the Geology of the Columbia River
A Self-Guided Tour of the Geology of the Columbia River Gorge— Portland Airport to Skamania Lodge, RESOURCES Stevenson, Washington by David K. Norman and Jaretta M. Roloff WASHINGTON DIVISION OF GEOLOGY AND EARTH RESOURCES Open File Report 2004-7 March 2004 NATURAL trip location DISCLAIMER Neither the State of Washington, nor any agency thereof, nor any of their em- ployees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any informa- tion, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or other- wise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the State of Washington or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the State of Washington or any agency thereof. WASHINGTON DEPARTMENT OF NATURAL RESOURCES Doug Sutherland—Commissioner of Public Lands DIVISION OF GEOLOGY AND EARTH RESOURCES Ron Teissere—State Geologist David K. Norman—Assistant State Geologist Washington Department of Natural Resources Division of Geology and Earth Resources PO Box 47007 Olympia, WA 98504-7007 Phone: 360-902-1450 Fax: 360-902-1785 E-mail: [email protected] Website: http://www.dnr.wa.gov/geology/ Cover photo: Looking east up the Columbia River Gorge from the Women’s Forum Overlook. Crown Point and its Vista House are visible on top of the cliff on the right side of the river. -

Life on Mars: Evidence from Martian Meteorites
Life on Mars: Evidence from Martian Meteorites David S. McKay1 Kathie L. Thomas-Keprta2 Simon J. Clemett2 Everett K. Gibson1, Jr., Lauren Spencer,1 and Susan J. Wentworth2. 1NASA Johnson Space Center, 2101 NASA Parkway, Houston TX USA; 2Jacobs Technology, NASA Johnson Space Center, 2101 NASA Parkway, Houston TX USA 77058 ABSTRACT New data on martian meteorite 84001 as well as new experimental studies show that thermal or shock decomposition of carbonate, the leading alternative non-biologic explanation for the unusual nanophase magnetite found in this meteorite, cannot explain the chemistry of the actual martian magnetites. This leaves the biogenic explanation as the only remaining viable hypothesis for the origin of these unique magnetites. Additional data from two other martian meteorites show a suite of biomorphs which are nearly identical between meteorites recovered from two widely different terrestrial environments (Egyptian Nile bottomlands and Antarctic ice sheets). This similarity argues against terrestrial processes as the cause of these biomorphs and supports an origin on Mars for these features. Keywords: Mars meteorites, ALH84001, Nakhla, Y000593, Life on Mars INTRODUCTION Martian meteorite ALH84001 Our original publication on this subject presented a suite of characteristics closely related in space and time within martian meteorite ALH84001, all of which could be best explained by a hypothesis that they were formed by microbes early in martian history.1 These observations included the presence of chemically zoned carbonates precipitated from water in cracks openings in the meteorite, morphological forms similar to known terrestrial fossils, polycyclic aromatic hydrocarbons (PAHs) associated with the carbonates, and nanophase magnetites embedded within the carbonates. -

Geology of the Central and Northern Parts of the Western Cascade Range in Oregon
Geology of the Central and Northern Parts of the Western Cascade Range in Oregon GEOLOGICAL SURVEY PROFESSIONAL PAPER 449 Prepared in cooperation with the State of Oregon, Departtnent of Geology and Mineral Industries Geology of the Central and Northern Parts of the Western Cascade Range in Oregon By DALLAS L. PECK, ALLAN B. GRIGGS, HERBERT G: SCHLICKER, FRANCIS G. WELLS, and HOLLIS M. DOLE ·~ GEOLOGICAL SURVEY PROFESSIONAL PAPER 449 Prepared in cooperation with the State of Oregon, Department of Geology and Mineral Industries ,... UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1964 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director . -~ The U.S. Geological Survey Library catalog card for this publication appears after page 56. For sale by the Superintendent of Documents,. U.S. Government Printing Office · · ·. Washington, D.C. 20402 CONTENTS Page Page Stratigraphy-Continued 1 Abstract------------------------------------------- Sardine Formation-Continued Introduction ______ --------------------------------- 2 Lithology and petrography-Continued Scope of investigation ______ - ___ - __ -------------- 2 Location, accessibility, and culture __ -------------- 2 Pyroclastic rocks __________ -- __ ---------'-- 33 Physical features ______ --_---_-_- ___ -----_------- 3 Age and correlation ____ - _--- __ -------------- 34 Climate and vegetation ___ --- ___ - ___ -----_------- 4 Troutdale Formation _____ ------------------------ 35 Fieldwork and reliability of the geologic -

Petrology of the I Volcanic Rocks of Guam
Petrology of the I Volcanic Rocks of Guam I GEOLOGICAL [SURREY PROFESSIONAL PAPER 403-C Petrology of the Volcanic Rocks of Guam By JOHN T. STARK With a section on TRACE ELEMENTS IN THE VOLCANIC ROCKS OF GUAM By JOSHUA I. TRACEY, JR., and JOHN T. STARK GEOLOGY AND HYDROLOGY OF GUAM, MARIANA ISLANDS GEOLOGICAL SURVEY PROFESSIONAL PAPER 403-C A study of the basaltic and andesitic volcanic rocks of Guam and their relations to similar rocks of Saipan and Japan UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1963 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.G. 20402 CONTENTS Page Page Abstract _________ ____________ _______ _________ Cl Rock type C8 Introduction _ _____ ________ _ _______ 1 Olivin 8 Acknowledgments _ _________ ____ ___ _____ 1 Basall 9 Outline of geology___ ___________ __ ___ 1 Hype]Hypersthene-bearing basalt-___ 10 Classification of the volcanic rocks__ _____ __ 2 AndesAndesite________________ __ 10 Mineralogy.- ______ _______ _________ __ _________ 5 HypeiHyperthene-bearing andesite.___ ___ 10 Plagioclase feldspar _______ _____ ____ _ _ _____ 5 Pyrocclastics__ ___ _______ 10 Alkali feldspar __ _____ ________ ____ ______ 5 \Water-laid tuffs and tuffaceous shales. __ __ 10 Pyroxene.. _ _____________ _ _____ ___ _ _____ 5 1Tuffaceous sandstones__ _ __ 11 Olivine_____________ _ ___ ___________ ______ 6 rPyroclastic conglomerate and breccia__________ 12 Silica minerals.. _____________________ _________ 6 Chemical composition and comparison with rocks of Ironores___ _________ __________ __ _________ 7 other areas_ar ____ _____ __ 12 Biotite- _____ ______ _________ _ __ _________ 7 PetrogenesPetrogenesis______________________ _____________ 26 Hornblende- _______ ____ ___ _______ _________ 7 Trace elenelements in the volcanic rocks of Guam, by Joshua _________ 7 I.