Policy for the Management of Extravasation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MASCC/ESMO ANTIEMETIC GUIDELINE 2016 with Updates in 2019
1 ANTIEMETIC GUIDELINES: MASCC/ESMO MASCC/ESMO ANTIEMETIC GUIDELINE 2016 With Updates in 2019 Organizing and Overall Meeting Chairs: Matti Aapro, MD Richard J. Gralla, MD Jørn Herrstedt, MD, DMSci Alex Molassiotis, RN, PhD Fausto Roila, MD © Multinational Association of Supportive Care in CancerTM All rights reserved worldwide. 2 ANTIEMETIC GUIDELINES: MASCC/ESMO These slides are provided to all by the Multinational Association of Supportive Care in Cancer and can be used freely, provided no changes are made and the MASCC and ESMO logos, as well as date of the information are retained. For questions please contact: Matti Aapro at [email protected] Chair, MASCC Antiemetic Study Group or Alex Molassiotis at [email protected] Past Chair, MASCC Antiemetic Study Group 3 ANTIEMETIC GUIDELINES: MASCC/ESMO Consensus A few comments on this guideline set: • This set of guideline slides represents the latest edition of the guideline process. • This set of slides has been endorsed by the MASCC Antiemetic Guideline Committee and ESMO Guideline Committee. • The guidelines are based on the votes of the panel at the Copenhagen Consensus Conference on Antiemetic Therapy, June 2015. • Latest version: March 2016, with updates in 2019. 4 ANTIEMETIC GUIDELINES: MASCC/ESMO Changes: The Steering Committee has clarified some points: 2016: • A footnote clarified that aprepitant 165 mg is approved by regulatory authorities in some parts of the world ( although no randomised clinical trial has investigated this dose ). Thus use of aprepitant 80 mg in the delayed phase is only for those cases where aprepitant 125 mg is used on day 1. • A probable modification in pediatric guidelines based on the recent Cochrane meta-analysis is indicated. -

Idamycin PFS® Idarubicin Hydrochloride Injection
Idamycin PFS® idarubicin hydrochloride injection Rx only FOR INTRAVENOUS USE ONLY WARNINGS 1. IDAMYCIN PFS Injection should be given slowly into a freely flowing intravenous infusion. It must never be given intramuscularly or subcutaneously. Severe local tissue necrosis can occur if there is extravasation during administration. 2. As is the case with other anthracyclines the use of IDAMYCIN PFS can cause myocardial toxicity leading to congestive heart failure. Cardiac toxicity is more common in patients who have received prior anthracyclines or who have pre- existing cardiac disease. 3. As is usual with antileukemic agents, severe myelosuppression occurs when IDAMYCIN PFS is used at effective therapeutic doses. 4. It is recommended that IDAMYCIN PFS be administered only under the supervision of a physician who is experienced in leukemia chemotherapy and in facilities with laboratory and supportive resources adequate to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity. The physician and institution must be capable of responding rapidly and completely to severe hemorrhagic conditions and/or overwhelming infection. 5. Dosage should be reduced in patients with impaired hepatic or renal function. (See DOSAGE AND ADMINISTRATION.) DESCRIPTION IDAMYCIN PFS Injection contains idarubicin hydrochloride and is a sterile, semi- synthetic, preservative-free solution (PFS) antineoplastic anthracycline for intravenous use. Chemically, idarubicin hydrochloride is 5, 12-Naphthacenedione, 9-acetyl-7-[(3- amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11- trihydroxyhydrochloride, (7S-cis). The structural formula is as follows: C26 H27 NO9 .HCL M.W. 533.96 1 Reference ID: 3668307 IDAMYCIN PFS is a sterile, red-orange, isotonic parenteral preservative-free solution, available in 5 mL (5 mg), 10 mL (10 mg) and 20 mL (20 mg) single-use-only vials. -

Combining Paclitaxel and Lapatinib As Second-Line Treatment for Patients with Metastatic Transitional Cell Carcinoma: a Case Series
ANTICANCER RESEARCH 32: 3949-3952 (2012) Combining Paclitaxel and Lapatinib as Second-line Treatment for Patients with Metastatic Transitional Cell Carcinoma: A Case Series STÉPHANE CULINE, ZINEB SELLAM, LINDA BOUAITA, ELIAS ASSAF, CATHERINE DELBALDO, MURIEL VERLINDE-CARVALHO and DAMIEN POUESSEL Department of Medical Oncology, Henri Mondor Hospital, Créteil, France Abstract. Background: Current first-line cisplatin-based trial comparing vinflunine with best supportive care (BSC) combination chemotherapy regimens provide interesting to BSC alone, an estimated difference in overall survival response rates but limited impact on survival for patients with (OS) of 2 months was reached in the intent-to-treat metastatic transitional cell carcinoma of the urothelium. Such population. However, a significant difference in OS was only results leave a significant patient population in need of salvage seen after removing patients who had major protocol therapy. Patients and Methods: As the epidermal growth factor violations (2). Therefore therapy for patients who fail first- receptors 1 and 2 (EGFR and HER2) are frequently line cisplatin-based chemotherapy remains a highly unmet overexpressed in urothelial carcinoma, we explored the medical need. feasibility of a combination of paclitaxel (80 mg/m2/week) and In a phase II study led by the French Genito-Urinary lapatinib (1,500 mg orally daily) for six patients who were Tumor group (GETUG), the activity of weekly paclitaxel as treated after failure of first-line platinum-based chemotherapy. second-line chemotherapy was assessed in 45 patients with Results: Only one out of six patients was able to receive the MTCCU. A low objective response rate (9%) along with a full doses during the first six weeks of treatment, while grade high rate of stabilization (38%) suggested limited impact as 2 or 3 diarrhea events required lapatinib dose reduction (one a single agent (3). -

Idarubicin.Pdf
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrIDARUBICIN idarubicin hydrochloride injection Preservative Free Solution, 1 mg/mL, intravenous injection Antineoplastic Agent Pfizer Canada ULC Date of Initial Authorization: 17,300 Trans-Canada Highway July 30, 2013 Kirkland, Quebec, H9J 2M5 Date of Revision: September 14, 2021 Submission Control Number: 255782 ©Pfizer Canada ULC, 2021 IDARUBICIN (idarubicin hydrochloride injection) Page 1 of 31 RECENT MAJOR LABEL CHANGES 7 Warnings and Precautions, Cardiovascular JA/2021 TABLE OF CONTENTS Sections or subsections that are not applicable at the time of authorization are not listed. RECENT MAJOR LABEL CHANGES ..........................................................................................2 TABLE OF CONTENTS ............................................................................................................2 1 INDICATIONS.............................................................................................................4 1.1 Pediatrics .................................................................................................................4 1.2 Geriatrics..................................................................................................................4 2 CONTRAINDICATIONS................................................................................................4 3 SERIOUS WARNINGS AND PRECAUTIONS BOX ...........................................................5 4 DOSAGE AND ADMINISTRATION................................................................................5 -

Summary Attachment for Eudract
Avenue E. Mounier 83/11 1200 Brussels Belgium Tel: +32 2 774 1611 Email: [email protected] www.eortc.org Summary Attachment for EudraCT Name of Individual study Table Referring to Part of the Dossier (For National Authority Use Sponsor/Company: Only) EORTC Name of the Volume: finished product Name of Active Page Ingredients: Clofarabine Cytarabine Idarubicin Title of the Study Clofarabine in combination with a standard remissioninduction regimen (AraC and idarubicin) in patients 18-60 years old with previously untreated intermediate and bad risk acute myelogenous leukemia (AML) or high risk myelodysplasia (MDS) : a phase I-II study of the EORTC-LG and GIMEMA (AML-14A trial) Investigators & Study Centers Number of Country City patients Belgium 4 101.Hopital Jules Bordet (BE) Brussels 1 109.A.Z. St Jan (BE) Brugge 3 Italy 28 3931.Tor Vergata Roma (IT) Roma 12 733.La Sapienza Ematologia (IT) Roma 16 Netherlands 43 304.RU Nijmegen (NL) Nijmegen 24 310.Univ Med Ctr Leiden (NL) Leiden 13 22.J Bosch 'S Hertogenb (NL) 's-Hertogenbosch 6 Grand Total 75 ST-006-AF-01 Page 1 of 4 Template version 2 Short Study Report for Health Authorities EORTC Name of Individual study Table Referring to Part of the Dossier (For National Authority Use Sponsor/Company: Only) EORTC Name of the Volume: finished product Name of Active Page Ingredients: Clofarabine Cytarabine Idarubicin Publication Willemze R, Suciu S, Muus P, Halkes CJ, Meloni G, Meert L, Karrasch M, Rapion J, (reference) Vignetti M, Amadori S, de Witte T, Marie JP.Clofarabine in combination with a standard remission induction regimen (cytosine arabinoside and idarubicin) in patients with previously untreated intermediate and bad-risk acute myelogenous leukemia (AML) or high-risk myelodysplastic syndrome (HR-MDS): phase I results of an ongoing phase I/II study of the leukemia groups of EORTC and GIMEMA (EORTC GIMEMA 06061/AML-14A trial). -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Monotherapy with Pixantrone in Histologically Confirmed Relapsed Or
research paper Monotherapy with pixantrone in histologically confirmed relapsed or refractory aggressive B-cell non-Hodgkin lymphoma: post-hoc analyses from a phase III trial Ruth Pettengell,1 Catherine Sebban,2 This post hoc analysis of a phase 3 trial explored the effect of pixantrone in Pier Luigi Zinzani,3 Hans Gunter patients (50 pixantrone, 47 comparator) with relapsed or refractory aggres- 4 5 Derigs, Sergey Kravchenko, Jack W. sive B-cell non-Hodgkin lymphoma (NHL) confirmed by centralized histo- 6 7 Singer, Panteli Theocharous, Lixia logical review. Patients received 28-d cycles of 85 mg/m2 pixantrone 7 8 Wang, Mariya Pavlyuk, Kahina M. dimaleate (equivalent to 50 mg/m2 in the approved formulation) on days Makhloufi8 and Bertrand Coiffier9,10 1, 8 and 15, or comparator. The population was subdivided according to 1St George’s University of London, London, UK, 2 previous rituximab use and whether they received the study treatment as Leon Berard Cancer Centre, Lyon, France, – 3Institute of Haematology “Le A Seragnoli”, 3rd or 4th line. Median number of cycles was 4 (range, 2 6) with pix- – University of Bologna, Bologna, Italy, 4St€adt Kli- antrone and 3 (2 6) with comparator. In 3rd or 4th line, pixantrone was Á Á nikum, Frankfurt-Hoeschest, Klinik fur€ Innere associated with higher complete response (CR) (23 1% vs. 5 1% compara- Medizin III, Frankfurt am Main, Germany, tor, P = 0Á047) and overall response rate (ORR, 43Á6% vs. 12Á8%, 5Chemotherapy and Intensive Treatment of Hae- P = 0Á005). In 3rd or 4th line with previous rituximab (20 pixantrone, 18 matology Diseases, Haematology Scientific Centre comparator), pixantrone produced better ORR (45Á0% vs. -
Transitional Cell Carcinoma: Options Beyond Nsaids Julie Marie Gillem, DVM, DACVIM (Oncology) Overview
Transitional Cell Carcinoma: Options Beyond NSAIDs Julie Marie Gillem, DVM, DACVIM (Oncology) Overview ✦ Background ✦ Surgical Options ✦ Pathology ✦ Medical Options ✦ Location and staging ✦ Radiation Therapy ✦ Behavior Options ✦ Etiology and risk factors ✦ Palliative care ✦ Work up and diagnosis ✦ What about cats? Objectives ✦ How do we determine when NSAIDs fail? ✦ When should we intervene with surgery, chemotherapy, radiation therapy, and additional palliative care? Pathology ✦ ~2% of canine cancer ✦ Invasive transitional cell carcinoma (TCC) most common ✦ Others: SCC, adenocarcinoma, undifferentiated carcinoma, rhabdomyosarcoma, fibroma, and other mesenchymal tumors Location and Staging ✦ TCC in dogs most often found in the trigone of the bladder ✦ Series of 102 dogs at PUVTH ✦ Urethra and bladder in 56% ✦ Prostate involvement in 29% male dogs ✦ Lymph node mets in 16% at diagnosis ✦ Distant mets in 14% at diagnosis ✦ Distant mets in 50% at death Location ✦ TCC in dogs most often is found in the trigone region of the bladder. ✦ In a series of dogs with TCC examined at the PUVTH, the tumor involved the urethra as well as the bladder in 57 of 102 dogs (56%), and it involved the prostate in 11 of 38 (29%) male dogs. WHO Staging ✦ 78% T2 tumors ✦ 20% T3 tumors Biological Behavior ✦ At diagnosis: ✦ Regional lymph node metastasis in 12-46 % (Norris et al 1992, Knapp et al 2000, Blackburn et al 2013) ✦ Distant metastasis in 16- 23% (Norris et al 1992, Blackburn et al 2013) ✦ Distant metastasis in 50% at death (Norris et al 1992, Knapp et al -

Oxaliplatin, 5-Fluorouracil and Leucovorin (FOLFOX) As Second- Line Therapy for Patients with Advanced Urothelial Cancer
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 7, No. 36 Clinical Research Paper Oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX) as second- line therapy for patients with advanced urothelial cancer Sheng Zhang1, Hongxi Xue2, Qiang Chen3 1Medical Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China 2Rizhao City Hospital of Traditional Chinese Medicine, Rizhao, China 3Department of Clinical Biochemistry, School of Public Health, Taishan Medical University, Tai’an, China Correspondence to: Sheng Zhang, email: [email protected] Keywords: urothelial cancer, oxaliplatin, leucovorin, 5-fluorouracil, clinical trial Received: February 08, 2016 Accepted: June 30, 2016 Published: July 07, 2016 ABSTRACT There is currently no standard treatment for metastatic urothelial cancer after failure of cisplatin-based therapy. The present retrospective study investigated the efficacy and safety of oxaliplatin plus 5-fluorouracil (5-FU) and leucovorin (LV) (FOLFOX) in locally advanced or metastatic urothelial cancer patients following cisplatin-based treatment. Thirty-three patients who had received one or two cisplatin-based regimens were treated with oxaliplatin (85 mg/m2) as a 2-h infusion on day 1, LV (200 mg/m2) as a 2-h infusion followed by bolus 5-FU (400 mg/m2) on day 1, or a 44-h continuous 5-FU (1,200 mg/m2) infusion. Patients were a mean of 67 years old with two involved organs. Metastases were mostly in the lung (43%), lymph nodes (51%) and liver (46%). Based on an intention-to-treat analysis, nine patients achieved a partial response, with an overall response rate of 27%. Eight (24%) patients had stable disease. -

Safe Handling of Cytotoxic, Monoclonal Antibody & Hazardous Non-Cytotoxic Drugs
PROCEDURE SAFE HANDLING OF CYTOTOXIC, MONOCLONAL ANTIBODY & HAZARDOUS NON-CYTOTOXIC DRUGS TARGET AUDIENCE All nursing, pharmacy and medical staff involved with dispensing, preparation, or administration of medicines. STATE ANY RELATED PETER MAC POLICIES, PROCEDURES OR GUIDELINES Administration and Management of Anti-Cancer Drugs Administration of Cytotoxics in the Home/Community Collection and Disposal of Soiled Linen Dangerous Goods and Hazardous Substances Environmental Management Individual Personal Protective Equipment (Cancer Research Division) Management of Cytotoxic Drug Spill Medication Management Medication Management for Nurses Pharmaceutical Review & Medication Supply Personal Protective Equipment Administration of Intravesical Immunotherapy BCG PURPOSE This procedure provides direction to all hospital staff involved in the management, preparation, transportation, administration of hazardous drugs and related wastes. In particular, safe handling practices for cytotoxic and hazardous non-cytotoxic drugs are outlined. BACKGROUND Hazardous drugs are regulated medicines that have been classified by the National Institute for Occupational Safety and Health (NIOSH) of the United States and/or the Cancer Institute New South Wales as posing a risk to health from occupational exposure. Exposure to hazardous drugs can result in adverse health effects in healthcare workers. The health risk depends on how much exposure a worker has to these drugs and the specific toxicity of the drug. The occupational exposure risk of hazardous drugs is therefore evaluated according to risk of internalisation (by ingestion, absorption through mucous membranes, and penetration of skin) and risk of toxicity (carcinogenicity, genotoxicity, teratogenicity, and reproductive or fertility impairment, organ toxicity) at low doses and continuous exposure. Hazardous drugs include both cytotoxic and non-cytotoxic medicines such as chemotherapy, monoclonal antibodies, immunomodulatory drugs, and some anti-infective drugs. -

How I Treat How I Treat Hyperleukocytosis in Acute Myeloid Leukemia
From www.bloodjournal.org by guest on January 2, 2016. For personal use only. How I Treat How I treat hyperleukocytosis in acute myeloid leukemia Christoph R¨olligand Gerhard Ehninger Medizinische Klinik und Poliklinik I, Universit¨atsklinikumder Technischen Universit¨at Dresden, Germany Hyperleukocytosis (HL) per se is a labora- chronic leukemias, and particularly leuko- lactate dehydrogenase as an indicator for tory abnormality, commonly defined by stasis occurs more often in acute myeloid high proliferation are part of prognostic a white blood cell count >100 000/mL, leukemia (AML) for several reasons. Only scores guiding risk-adapted consolidation caused by leukemic cell proliferation. Not a small proportion of AML patients present strategies, HL at initial diagnosis must be the high blood count itself, but complica- with HL, but these patients have a partic- considered a hematologic emergency and tions such as leukostasis, tumor lysis syn- ularly dismal prognosis because of (1) a requires rapid action of the admitting drome, and disseminated intravascular higher risk of early death resulting from physician in order to prevent early death. coagulation put the patient at risk and HL complications; and (2) a higher proba- (Blood. 2015;125(21):3246-3252) require therapeutic intervention. The risk bility of relapse and death in the long run. of complications is higher in acute than in Whereas initial high blood counts and high Incidence and pathophysiology In untreated acute myeloid leukemia (AML), ;5% to 20% of patients factor VII.18 TLS may occur as a result of spontaneous or treatment- present with hyperleukocytosis (HL).1-10 In a patient with HL, under- induced cell death. -
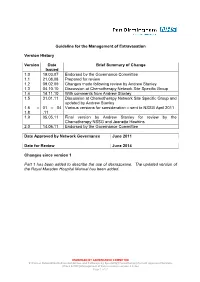
Guideline for the Management of Extravasation
Guideline for the Management of Extravasation Version History Version Date Brief Summary of Change Issued 1.0 19.03.07 Endorsed by the Governance Committee 1.1 21.08.08 Prepared for review 1.2 09.02.09 Changes made following review by Andrew Stanley 1.3 04.10.10 Discussion at Chemotherapy Network Site Specific Group 1.4 14.11.10 With comments from Andrew Stanley 1.5 31.01.11 Discussion at Chemotherapy Network Site Specific Group and updated by Andrew Stanley 1.6 – 01 – 04 Various versions for consideration – sent to NSSG April 2011 1.8 .11 1.9 05.05.11 Final version by Andrew Stanley for review by the Chemotherapy NSSG and Jeanette Hawkins 2.0 14.06.11 Endorsed by the Governance Committee Date Approved by Network Governance June 2011 Date for Review June 2014 Changes since version 1 Part 1 has been added to describe the use of dexrazoxane. The updated version of the Royal Marsden Hospital Manual has been added. ENDORSED BY GOVERNANCE COMMITTEE S:\Cancer Network\Guidelines\Guidelines and Pathways by Speciality\Chemotherapy\Current Approved Versions (Word & PDF)\Management of Extravasation version 2.0.doc Page 1 of 21 1 Scope of the Guideline This guidance has been produced to support the following: The prevention of the extravasation of intravenous anti-cancer drugs. The early detection of the extravasation of intravenous anti-cancer drugs. The treatment of the extravasation of intravenous anti-cancer drugs. 2 Guideline Statement Statement 2 The Network Site Specific Group has agreed to adopt the Royal Marsden Hospital Manual of Clinical Nursing Procedures 7th Edition; Blackwell Publishing (2008), chapter on extravasation, with the addition of a section on dexrazoxane.