Zooplankton Community Structure in Pengkalan Chepa River Basin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
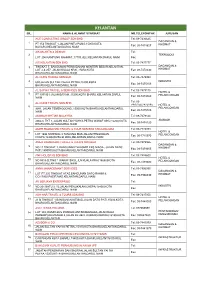
Kelantan Bil
KELANTAN BIL. NAMA & ALAMAT SYARIKAT NO.TELEFON/FAX JURUSAN ACE CONSULTING GROUP SDN BHD Tel: 09-7436625 DAGANGAN & 1 PT 153 TINGKAT 1,JALAN PINTU PONG,15000,KOTA Fax: 09-7418827 KHIDMAT BAHARU,KELANTAN,DARUL NAIM AIKON ARTS & DESIGN Tel: 2 TEKNOLOGI LOT 206 KAMPUNG RAHMAT,,17700,JELI,KELANTAN,DARUL NAIM Fax: AIR KELANTAN SDN BHD Tel: 09-7437777 DAGANGAN & 3 TINGKAT 5, BANGUNAN PERBADANAN MENTERI BESAR,KELANTAN, LOT 2 & 257, JALAN KUALA KRAI,,15050,KOTA Fax: 09-7472030 KHIDMAT BHARU,KELANTAN,DARUL NAIM AL QUDS TRAVEL SDN BHD Tel: 09-7479999 4 650,JALAN SULTAN YAHYA PETRA,15200,KOTA INDUSTRI Fax: 09-7475105 BHARU,KELANTAN,DARUL NAIM AL SAFWA TRAVEL & SERVICES SDN BHD Tel: 09-7475115 HOTEL & 5 PT 1971-B1 JALAN BAYAM,,15200,KOTA BHARU,KELANTAN,DARUL Fax: 09-7479060 PELANCONGAN NAIM Tel: 09- AL-QUDS TRAVEL SDN BHD 7475155/7475145 HOTEL & 6 9981, JALAN TEMENGGONG,,15000,KOTA BHARU,KELANTAN,DARUL PELANCONGAN Fax: 09-7475105 NAIM AMANAH IKHTIAR MALAYSIA Tel: 09-7478124 7 2002-C TKT 1,,JALAN SULTAN YAHYA PETRA WAKAF SIKU,15200,KOTA AMANAH Fax: 09-7478120 BHARU,KELANTAN,DARUL NAIM AMER RAMADHAN TRAVEL & TOUR SDN BHD TANJUNG MAS Tel: 09-7715973 HOTEL & 8 LOT 1894 SIMPANG 3 TANJUNG MAS,JALAN PENGKALAN Fax: 09-7715970 PELANCONGAN CHEPA,15300,KOTA BHARU,KELANTAN,DARUL NAIM AMER RAMADHAN TRAVEL & TOURS SDN BHD Tel: 09-7479966 DAGANGAN & 9 NO 11 TINGKAT 1, BANGUNAN TH,KOMPLEKS NIAGA , JALAN DATO' Fax: 09-7479955 KHIDMAT PATI,1500000,KOTA BHARU,KELANTAN,DARUL NAIM ANF HOLIDAYS SDN BHD Tel: 09-7488600 HOTEL & 10 NO 5515-D,TING 1 WAKAF SIKU,,JLN KUALA -

Branches/Self-Service Machines in Flood Affected Areas
ATTACHMENT (COMMERCIAL BANKS) BRANCHES/SELF-SERVICE MACHINES IN FLOOD AFFECTED AREAS Note: ATM - Automated Teller Machine CRM - Cash Recycling Machine CDM - Cash Deposit Machine CQM - Cheque Deposit Machine Kelantan Functioning ATM/CDM Location/Address of Branch Town Location/Address of Branch No Bank (Incapacitated and not in operation) Location/Address 1 Affin Bank Berhad NO 3788 H–I Jalan Sultan Ibrahim 15050 Kota Bharu, - Branch Kelantan Kota Bharu Kem Desa Pahlawan. 16500, - Offsite Kota Bharu, Kelantan A1 & A2, Block A, Bandar Baru Bukit Bunga, 17700 Jeli Bukit Bunga, Tanah Merah, - Branch Kelantan 2 Alliance Bank Malaysia - - - - Berhad Page 1 of 25 BRANCHES/SELF-SERVICE MACHINES IN FLOOD AFFECTED AREAS Note: ATM - Automated Teller Machine CRM - Cash Recycling Machine CDM - Cash Deposit Machine CQM - Cheque Deposit Machine Kelantan Functioning ATM/CDM Location/Address of Branch Town Location/Address of Branch No Bank (Incapacitated and not in operation) Location/Address 3 AmBank (M) Berhad Branch Branch 13 ATMs at 7 Eleven outlets:- 1) Jalan Raja Perempuan Zainab 2 2) Jalan Tok Kenali 3) Tanjung Chat Ground & First Floor, Lot 343 4) Pasir Tumboh Section 13, Jalan Sultan 5) Kok Lanas Kota Bharu - Ibrahim, 15000 Kota Bharu, 6) Pasir Pekan Offsite Kelantan 7) Taman Muda Murni 8) Wakaf Baru 9) Padang Tembak 10) Kota Jemba 11) Panji 12) Jalan Hospital 13) Pantai Cahaya Bulan 1 ATM at Adventa, Pengkalan Chepa Offsite 1 ATM at Tesco Kota Bahru 1 ATM at Mydin Kubang Lot 151, Jalan Masjid Lama, Pasir Mas - Branch 17000 Pasir Mas, Kelantan -

Direktori Pegawai Farmasi Pusat Rtg Methadone
DIREKTORI PUSAT RTG METHADONE BAHAGIAN PERKHIDMATAN FARMASI KEMENTERIAN KESIHATAN MALAYSIA Edisi September 2010 KANDUNGAN PUSAT RAWATAN NEGERI PERLIS ............................................................................................................ 4 1. HOSPITAL ......................................................................................................................................... 4 2. KLINIK KESIHATAN ........................................................................................................................... 4 PUSAT RAWATAN NEGERI KEDAH ............................................................................................................ 5 1. HOSPITAL ......................................................................................................................................... 5 2. KLINIK KESIHATAN .......................................................................................................................... 6 3. PENJARA .......................................................................................................................................... 9 4. AGENSI ANTI DADAH KEBANGSAAN ............................................................................................. 10 PUSAT RAWATAN NEGERI PULAU PINANG ............................................................................................ 11 1. HOSPITAL ....................................................................................................................................... 11 2. KLINIK -

Senarai GM Kelantan
BIL GIATMARA ALAMAT TELEFON & FAKS KURSUS YANG DITAWARKAN Wisma Amani, Lot PT 200 & 201, 09-7422990 (Am) Pejabat GIATMARA Negeri Taman Maju, Jalan Sultan Yahya Petra, 09-7422992 (Faks) 15200 Kota Bharu, Kelantan Darul Naim PENDAWAI ELEKTRIK (PW2) 09-7787311, PENDAWAI ELEKTRIK (PW4 - 36 BULAN) 1 Bachok (4) Lot 665, Kampung Serdang Baru, 16310 Bachok 09-7787312 (F) TEKNOLOGI AUTOMOTIF FASHION AND DRESSMAKING INDUSTRIAL MAINTENANCE 09-9285171, 2 Gua Musang (3) Felda Chiku 5, 18300 Gua Musang TEKNOLOGI MOTOSIKAL 09-9287637 (F) TEKNOLOGI AUTOMOTIF PENDAWAI ELEKTRIK (PW2) 09-9468553, FASHION AND DRESSMAKING 3 Jeli (4) Kampung Rahmat, 17700 Ayer Lanas 09-9468550 (F) TEKNOLOGI AUTOMOTIF TEKNOLOGI BAIKPULIH & MENGECAT KENDERAAN FASHION AND DRESSMAKING HIASAN DALAMAN 09-7880211, 4 Ketereh (5) Lot 236, Kuarters KADA Ketereh, 16450 Ketereh SENI SULAMAN KREATIF 09-7880212 (F) SENI SULAMAN KREATIF (SULAMAN MESIN) SENI SULAMAN KREATIF (SULAMAN TANGAN) PENDAWAI ELEKTRIK (PW2) PENDAWAI ELEKTRIK (PW4 - 12 BULAN) 5 Kota Bharu (4) Jalan Telipot, 15150 Kota Bharu 09-7447058 (P/F) TEKNOLOGI AUTOMOTIF TEKNOLOGI ELEKTRONIK AUDIO VISUAL 09-9362689, TEKNOLOGI MOTOSIKAL 6 Kuala Krai (2) Kampung Jelawang, 18200 Dabong, Kuala Krai 09-9361689 (F) FASHION AND DRESSMAKING Lot 2399 Kg Padang Bongor, Kubang Kerian, 16150 CONFECTIONARY AND BAKERY Kota Bharu 09-7666871, 7 Kubang Kerian (3) FASHION AND DRESSMAKING 09-7666872 (F) SOLEKAN DAN TERAPI KECANTIKAN TEKNOLOGI AUTOMOTIF 09-9750016, TEKNOLOGI ELEKTRONIK AUDIO VISUAL 8 Machang (4) Balai Polis Lama, 18500 Machang 09-9750017 -

Senarai Balai Bomba Dan Penyelamat Seluruh Malaysia
SENARAI BALAI BOMBA DAN PENYELAMAT DI SELURUH MALAYSIA Bil Negeri Zon Balai Alamat Perhubungan 1 WP Putrajaya - BBP Putrajaya Balai Bomba Dan Penyelamat Putrajaya, Lebuh Wawasan, Tel:03-88880970 / 0971 Presint 7, 62250 Putrajaya ,WP Putrajaya Fax:03-88880019 Email: [email protected] 2 Johor Zon Johor Bharu BBP Johor Bahru Jalan Tun Abdul Razak 80648 Johor Bahru , Johor Tel:07-2243444 Fax:07-2244344 Email: [email protected] 3 Johor Zon Johor Bharu BBP Johor Jaya Jalan Ros Merah 4/1, Johor Jaya, 81100 Johor Bahru, Johor Tel:07-3511444 Fax:07-3515344 Email: [email protected] 4 Johor Zon Johor Bharu BBP Pasir Gudang Jalan Mesjid Pasir Gudang 81700, Johor Tel:07-2513444 / 2513944 Fax:07-2532985 Email: bbp- 5 Johor Zon Johor Bharu BBP Kulai Jalan Pejabat Kerajaan Kulaijaya 81000, Johor Tel:07-6634444 Fax:07-6621121 Email: [email protected] 6 Johor Zon Johor Bharu BBP Pontian Jalan Alsagoff Pontian 82000, Johor Tel:076864444,076874444 Fax:07-6879877 Email: [email protected] 7 Johor Zon Johor Bharu BBP Pekan Nenas Jalan Pontian Pekan Nanas 81648, Johor Tel:07-6994444 Fax:07-6993345 Email: bbp- 8 Johor Zon Johor Bharu BBP Pontian Baru Jalan Sekolah Arab Pontian 82000, Johor Tel:076864044, 076866044 Fax:076869044 Email: 9 Johor Zon Johor Bharu BBP Pulai Jalan Kebudayaan 22, Taman Universiti, Skudai, 81300, Johor Tel:07-5204144 Fax:07-5205944 Email: [email protected] 10 Johor Zon Johor Bharu BBP Kulai Jaya Jalan Putra 1, Kulai Jaya 81000, Johor Tel:07 - 590 8711 Fax:07 -

Members of EAST COAST AUTOMOBIL REPAIR ASSOCIATION
Members of EAST COAST AUTOMOBIL REPAIR ASSOCIATION Workshop's Name Workshop's Address Postcode Office Tel Office Fax Lot 62, Batu 3, Wakaf Che Yeh, Jalan Kuala Krai, 15050 Kota Juara Motor Works 15050 Kelantan 7212412 Bharu, Kelantan. Loong Sheng Motor Workshop 7841, Jalan Salor, Wakaf Che Yeh, 15100 Kota Bharu, Kelantan. 15100 Kelantan 7448029 7470058 Eastern Auto Services Sdn Bhd 5721, Kawasan Meil Lundang, 15200 Kota Bharu, Kelantan. 15200 Kelantan 7436228 7436229 Hup Soon Motor Sdn Bhd Lot 44, Kawasan Miel Landang, 15200 Kota Bharu, Kelantan. 15200 Kelantan 7441607 7432848 Jaya Motor Work 1792, Jalan Dato Lundang, 15200 Kota Bharu, Kelantan. 15200 Kelantan 7444755 7482912 Loo Motors 1212-G, Jalan Long Yunus, 15200 Kota Bharu, Kelantan. 15200 Kelantan 55A, Jalan Cabang 4, Bayam Guchil, Kg. Bayam, 15200 Kota Yakin Pulih Workshop 15200 Kelantan 7478177 7472458 Bharu, Kelantan. Chua & Sons Motors 2800-C, Jalan Pengkalan Chepa, 15400 Kota Bharu, Kelantan. 15400 Kelantan 7449327 7447327 Lot 1209, Jalan Pasir Puteh, Kubang Kerian, 16100 Kota Bharu, Lee Motor Work 16100 Kelantan 7653337 Kelantan. Lot 1683, Mukim Tapang, Panji, Kg.Dusun Raja, 16100 Kota Sim Motor Workshop (KB) Sdn Bhd 16100 Kelantan 7730199 7744199 Bharu, Kelantan. Lot 1702, Batu 6 1/4, Kg. Kedai Mulong, 16010 Kota Bharu, Dasar Vila (M) Sdn Bhd 16010 Kelantan Kelantan. Lot 2743, Batu 5 1/2, Kampung Tunjong, Jalan Kuala Krai, 16010 SH Kota Bharu Workshop Sdn Bhd 16010 Kelantan 7126026 7126384 Kota Bharu, Kelantan. Lot 4102, Kawasan Perindustrian Pengkalan Chepa, Padang PTM Pertama Motor Sdn Bhd 16100 Kelantan 7713888 7713887 Tembak, 16100 Kota Bharu, Kelantan. Lot 460, Kampung Padang Demit, Jalan Raja Perempuan Zainab Sabri Motor Workshop 16150 Kelantan 7656073 7651073 2, Sek 51, Kubang Kerian, 16150 Kota Bharu, Kelantan. -

Senarai Kedai Yang Mengambil Bahagian (26 Jul 2021)
NESTLÉ PROFESSIONAL RASA-RASA MALAYSIA SENARAI KEDAI YANG MENGAMBIL BAHAGIAN (26 JUL 2021) NAMA KEDAI NEGERI ALAMAT JOHOR DE MAS BAKERY JOHOR NO.23GF JL HARMONIUM 23/12 ,TAMAN DESA FONG J FOOD AND BEVERAGE JOHOR TEBRAU,JOHOR BAHRU JOHOR NO.65 JALAN RIANG UTAMA ,TAMAN GEMBIRA GC BAKERY & CAFE JOHOR ,TAMPOI,JOHOR GOOD TIME GOOD DAY HUB JOHOR NO.22 GROUND FLOOR ,JALAN UTAMA 2/4 ,BANDAR GOOD TIME GOOD DAY HUB JOHOR UTAMA ,85000 SEGAMAT JOHOR, HW FOOD INDUSTRY JOHOR LOT 1368 JALAN GENUANG MUKIM SUNGAI SEGAMAT HW FOOD INDUSTRY JOHOR ,85000 SEGAMAT JOHOR NO.26 & 28 JALAN KUNING ,TAMAN BUKIT PASIR ,83000 JA SING RESTAURANT SDN. BHD. JOHOR BATU PAHAT JOHOR KAFE RASA RIA JOHOR NO.21 JALAN TEMIANG ,TAMAN MAKMUR ,83000 BATU KEDAI KOPI SATU RINGGIT JOHOR PAHAT JOHOR NORTH STATION JOHOR NO.97 ,JALAN CAHAYA BARU 1 ,TAMAN BCB ,83700 YONG NORTH STATION JOHOR PENG ,JOHOR OCEANMAN BBQ STEAM BOAT JOHOR NO.118-120 JALAN FLORA UTAMA 8 ,TAMAN FLORA PREMIUM WOK F&B ENTERPRISE JOHOR UTAMA ,83000 BATU PAHAT JOHOR PTD 68948 BATU 7 1/2,JALAN KOTA TINGGI,81100 JOHOR PRET A MANGER SDN BHD JOHOR BAHRU RESTORAN HOCK MUN JOHOR 3 JALAN TEMBIKAI ,86000 KLUANG JOHOR,,, LG 56 LOWER GRD THE SUMMIT ,NO.88 JALAN BAKAU RESTORAN VK JOHOR CONDONG ,83000 BATU PAHAT JOHOR,, SAN YUAN GOURMET HOUSE JOHOR NO.1 JALAN SETIA JAYA UTAMA ,TAMAN SETIA JAYA ,83000 SAN YUAN GOURMET HOUSE BP JOHOR BATU PAHAT JOHOR FC-001-002-003A ,KIP MART TAMPOI ,PTD 129842 JALAN SELERA FOOD LINK ENTERPRISE JOHOR SKUDAI LAMA ,TAMAN TAMPOI INDAH ,JOHOR. -

PDF File Generated From
OCCASION This publication has been made available to the public on the occasion of the 50th anniversary of the United Nations Industrial Development Organisation. DISCLAIMER This document has been produced without formal United Nations editing. The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations Industrial Development Organization (UNIDO) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries, or its economic system or degree of development. Designations such as “developed”, “industrialized” and “developing” are intended for statistical convenience and do not necessarily express a judgment about the stage reached by a particular country or area in the development process. Mention of firm names or commercial products does not constitute an endorsement by UNIDO. FAIR USE POLICY Any part of this publication may be quoted and referenced for educational and research purposes without additional permission from UNIDO. However, those who make use of quoting and referencing this publication are requested to follow the Fair Use Policy of giving due credit to UNIDO. CONTACT Please contact [email protected] for further information concerning UNIDO publications. For more information about UNIDO, please visit us at www.unido.org UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Tel: (+43-1) 26026-0 · www.unido.org · [email protected] 16058 • SMALL SCALE INDUSTRIAL DEVELOPMENT IN MALAYSIA: THE CASE OF THE 'NURSERY FACTORY SCHC.ME' IN PENGKALAN CHtPA, KELANTAN by Anuwar Ali & Ismail M. -

Senarai Premis Penginapan Pelancong : Kelantan 1
SENARAI PREMIS PENGINAPAN PELANCONG : KELANTAN BIL. NAMA PREMIS ALAMAT POSKOD DAERAH 1 Crystal Lodge 124, Jalan Che Su 15000 KOTA BHARU 2 Sutera Inn Prima Jalan Dusun Muda, Off Jalan Bayam 15200 KOTA BHARU 3 KESEDAR HOTEL TRAVEL & TOURS Jalan Kesedar Inn 18300 Gua Musang 4 Suria Hotel Lot 254-256, Jalan Padang Garong 15000 KOTA BHARU 5 H.B Village Resort Pantai Cahaya Bulan 15350 KOTA BHARU 6 Hotel Usaha 187, Jalan Pantai 18300 Gua Musang 7 Grand Riverview Hotel Jalan Post Office Lama 15000 KOTA BHARU 8 Renaissance Kota Bharu Hotel Kota Sri Mutiara, Jalan Sultan Yahya Petra 15150 KOTA BHARU 9 Paloh Inn Wisma Nordin, Lot 74, Kg.Paloh, Pintu Geng 15050 KOTA BHARU 10 Dynasty Inn 2865-D & E, Jalan Sultanah Zainab 15050 KOTA BHARU 11 Irama Timur Resort House 6800-D, Jalan Bayam Lama 15200 KOTA BHARU 12 Sabrina Court Hotel Lot 171-181, Jalan Padang Garong 15000 KOTA BHARU 13 Sutera Calan Lot 1911, 1912, 1957, Kg.Bandar Taway 16300 Bachok 14 d'Mawar Chalet Taman Tasik Sri 17500 Tanah Merah 15 Fully Inn 75, Jalan Pekan Lama 18300 Gua Musang 16 Sudara Beach Resort Lot 128, Mukim Telong,Jln Tok Bali 16300 Bachok 17 Z & H Inn Lot 2731, Paya Rambai, Jln. Bayam 15200 KOTA BHARU 18 D Muara Chalet Lot 3067 Kg Sg.Dua Melati Repek 16300 Bachok 19 Flora Place Lot 202 Jalan Kebun Sultan 15350 KOTA BHARU 20 Pasir Belanda Resort Kg Lipat Sanggul, Kg Banggol 15350 KOTA BHARU 21 Ridel Hotel Kota Bharu Lot A-05-01, Jalan Pasar Lama, Seksyen 9 15000 KOTA BHARU 22 Royal Guest House Lot 440 & 443, Lot 448-452, Jln Hilir Kota 15300 KOTA BHARU 23 Bayu Melawi Chalet Lot 3066, Kg. -

Co Serving Lojing Highlands for Sustainable Development
CO SERVING LOJING HIGHLANDS FOR SUSTAINABLE DEVELOPMENT Editors Ibrahim Che Omar Zulhazman Hamzah TABLE OF CONTENT CONTENT PAGE Distributed by : PREFACE Unit Penerbitan Universiti Malaysia Kelantan SECnON I :GENERAL Universiti Malaysia Kelantan, 1 Karung Berkunci 36, Pengkalan Chepa, 16100, Conservation of Lojing Highlands: The Role of Education and Research Ibrahim Che Omar Kota Bharu, Kelantan, Malaysia. Lojing Highlands: To Conserve or Not to Conserve? 15 MafYC1tiMohamed and Mohd. Noh Dafimin C Penerbit Universiti Malaysia Kelantan, 2010 The Importance of Gua Musang - LOjing as an Integrated Nature Tourism Belt 26 Robert Francis Peters Perpustakaaan Hegara Malaysia The Lojing Highlands: A Potential Nature Tourism Attraction 31 Ibrahim Che Omar Danny I.W. Chew and Zulhazman Hamzah Conserving Lojing Highlands For Sustainable Development / Ibrahim Che Omar, Zufhazman Hamzah. In-Situ Water Quality Measurements of Streams in Lojing Highlands, Kelantan 39 Sahana HanJn and Saharah Ibrahim . ISBN 978-983-44043-7-6 SEcnON II:FlORA i) Lojing Highlands ii) Sustainable Development iii) Zulhazman Hamzah Spatial Distribution and Conservation of Raffiesia kerr;; in Lojing Highlands, Kelantan 44 Zulhazman Hamzah, MafYC1tiMohammed, Comelius Peter and Penerbit Universiti Malaysia Kelantan Mohd Mahmud@Mansur Locked Bag 36, Pengkalan Chepa, 16100 Kota Bharu, Ke/antan, Malaysia. Mosses of Lojing Highlands, Kelantan 55 Monica Sulelman, Ahmad Damanhuri, Yong Kien- Thai and Mohamad Ruzi Abd Rahman Preliminary Survey on Pteridophytes in Lojing Highlands, Kelantan -

Morphy Richard Hot Water Dispenser (292776) Service Centre Listing
Morphy Richard Hot Water Dispenser (292776) Service Centre Listing Walk into Service Location Customer Care Centre Centre Address Tel no. PPS PJ Yes 11a, Jalan 223, Section 51a, 46100 Petaling Jaya, Selangor. 1800-881-770 Central PPS PJ Yes 11a, Jalan 223, Section 51a, 46100 Petaling Jaya, Selangor. 1800-881-770 PPS PJ Yes 11a, Jalan 223, Section 51a, 46100 Petaling Jaya, Selangor. 1800-881-770 PPS Bkt Minyak Yes 1165, Lorong Perindustrian Bukit Minyak 16, Tmn Perindustrian Bukit Minyak, 14100 Simpang Ampat, Pulau Pinang. 04-5070393 PPS Bkt Minyak Yes 1165, Lorong Perindustrian Bukit Minyak 16, Tmn Perindustrian Bukit Minyak, 14100 Simpang Ampat, Pulau Pinang. 04-5070393 PANATEC SERVICE CENTER Yes 8, JALAN TASEK TIMUR,TAMAN TASEK INDRA,31400 IPOH, PERAK. 05-5453755 Northern ISK ELECTRICAL SERVICE Yes NO. 73, JALAN RISHAH PERMAI 1, TAMAN RISHAH PERMAI,(DEPAN MASJID TAMAN MAS), 30100 IPOH, PERAK. 019-5611088 Keat Radio Yes 143, Perak Road, 10150 Penang. 04-2269923 PPS Bkt Minyak Yes 1165, Lorong Perindustrian Bukit Minyak 16, Tmn Perindustrian Bukit Minyak, 14100 Simpang Ampat, Pulau Pinang. 04-5070393 PPS Bkt Minyak Yes 1165, Lorong Perindustrian Bukit Minyak 16, Tmn Perindustrian Bukit Minyak, 14100 Simpang Ampat, Pulau Pinang. 04-5070393 PPS JB Yes 31, Jalan Ros Merah 1/1, Taman Johor Jaya, 81100 Johor Bahru, Johor. 07-3556262 Daily Electronic Yes 95, Jalan Merah 5, Taman Mas Merah, 84000 Muar, Johor. 017-3629609 PPS JB Yes 31, Jalan Ros Merah 1/1, Taman Johor Jaya, 81100 Johor Bahru, Johor. 07-3556262 Southern Daily Electronics Service Trading Yes 95, Jalan Merah 5, Taman Mas Merah, 84000 Muar, Johor. -

Peta Panduan Jalan KELANTAN Ibu Negeri
PETUNJUK Peta Panduan Jalan KELANTAN Ibu Negeri Daerah 101º 20’E 1 101º 30’E 2 101º 40’E 3 101º 50’E 4 102º 00’E 5 102º 10’E 6 102º 20’E 7 102º 30’E 8 102º 40’E Bazar Tengku Anis Pekan Hentian Bas ke PCB GG Garden Hostel Istana Nasi R JLN. DUSUN RAJA N A Batu Ulam Payang Serai LAUT CHINA A Tourism Khalifa Jln. Maahad R Apartment Malaysia Star Family Kota Timur Hostel Kg. Kraf Tambatan Muzium Sempadan Antarabangsa di Raja Royal Tangan & E’ E Inn Perang Muzium Masjid Guesthouse Muzium R Sri Chiang Mai Islam Muhammadi Feri ke Menara Jln. HilirKraf Kota SELATAN Kampung Tinjau Ideal China Town Laut Pasar Malam Guesthouse Padang Merdeka Istana Oriental Kopitiam Jahar R Kampung Pantai 6º 15’N Pantai Seri Tujuh 6º 15’N Sempadan Negeri Ridel Jln. Tengku Besar Istana Jln. Sekolah Merbau Crown Garden Suri Homestay Pengkalan Kok Majid Jetty Money Changer Dynasty Balai Besar Arena Seni Suara Wat Machimmaram KFC JLN. KEBUN SULTAN Burung Indah Pantai Juite Pantai Cahaya Bulan Timur R Mydin Inn Sempadan Daerah Pasar Besar Pengkalan Kubor Bazar JLN. PINTU PONG Jln. Pasar Lama Siti Khadijah Pantai Kuala Pak Amat Jln. Tengku Chik Buluh Kubu R Jln. Seri Cemerlang Wat Mai Suvankhiri 134 Tumpat Mc’Donalds JLN. BULUH KUBU MGU Pantai Sabak B Muzdalfa Fried R Firdaus B CELCOMWat Centre Photivihan TUMPAT Jalanraya Persekutuan Jln. Tg. Putera Jln. Tok Hakim Chicken Flora Temenggong Place KB Mutiara Inn Sultan Ismail Petra Airport Parkson/ Giant Mydin R FourMakam Season Tok Janggut Suntwo R MAXIS Centre Sri Cemerlang Grand The Riverview Hostel Pengkalan Chepa KFC Store Sabrina KOTA BHARU R Court Wakaf Bharu Nombor Jalan Persekutuan Pizza JLN.