List Item Draft Assessment Report on Fumaria Officinalis L., Herba
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
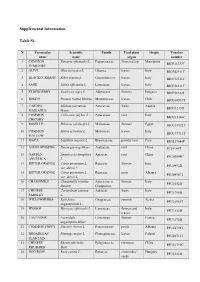
Supplemental Information Table
Supplemental Information Table S1. N Vernacular Scientific Family Used plant Origin Voucher name name organ number 1 COMMON Fumaria officinalis L. Papaveraceae flowered top Macedonia BIOF26733T FUMITORY 2 OLIVE Olea europaea L. Oleacee leaves Italy BIOF42911T 3 BLACKCURRANT Ribes nigrum L. Grossulariacee leaves Italy BIOF52511T 4 SAGE Salvia officinalis L. Lamiaceae leaves Italy BIOF56111T 5 ELDERBERRY Sambucus nigra L. Adoxaceae flowers Bulgaria BIOF56322I 6 BOLDO Peumus boldus Molina Monimiaceae leaves Chile BIOU08511T 7 CARDUS Silybum marianum Asteraceae fruits Austria BIOU12155I MARIANUS Gaert. 8 COMMON Cichorium intybus L. Asteraceae root Italy BIOU15344C CHICORY 9 ROSELLE Hibiscus sabdariffa L. Malvaceae flowers Egypt BIOU31922T 10 COMMON Malva sylvestris L. Malvaceae leaves Italy BIOU37311T MALLOW 11 MACA Lepidium meyenii L. Brassicaceae powder root Peru BIOZ37044P 12 ASIAN GINSENG Panax ginseng Meyer Araliaceae root China PC29144T 13 GARDEN Angelica archangelica Apiaceae root China PFU03844C ANGELICA L. 14 BITTER ORANGE Citrus aurantium L. Rutaceae flowers Iran PFU04922I var. dulcis L. 15 BITTER ORANGE Citrus aurantium L. Rutaceae zests Albania PFU04967T var. dulcis L. 16 CHAMOMILE Chamomilla recutita Asteraceae o flowers Italy PFU10522I Rausch. Compositae 17 CHINESE Coriandrum sativum Apiaceae fruits Italy PFU17955I PARSLEY L. 18 WILLOWHERBS Epilobium Onagraceae summit Serbia PFU21933T angustifolium L. 19 HYSSOP Hyssopus officinalis L. Lamiaceae flowers and Italy PFU31322I leaves 20 LAVENDER Lavandula Lamiaceae flowers France PFU32722I angustifolia Miller 21 COMMON POPPY Papaver rhoeas L. Papaveraceae petals Albania PFU44708T 22 BROADLEAF Plantago major L. Plantaginaceae leaves Poland PFU46711T PLANTAIN 23 CHINESE Rheum officinale Polygonaceae rhizomes China PFU51799C RHUBARB Baill. 24 DOG ROSE Rosa canina L. Rosaceae cinorrodes/ Hungary PFU53310I seeds 25 RUSTYBACK Ceterach officinarum Aspleniaceae aerial parts Italy PFU59533T DC. -

Spring in North Cyprus
Spring in North Cyprus Naturetrek Tour Report 1 - 8 April 2016 Cyprus Wheatear beema Yellow Wagtail Eastern Festoon Masked Shrike Report compiled by Andy Harding and Jessica Turner Images by Andy Harding Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Spring in North Cyprus Tour participants: Andy Harding and Jessica Turner (leaders) and Turgay Azizoglu (local guide) with 16 Naturetrek clients Day 1 Friday 1st April The tour started with an early flight from Heathrow T5. We arrived at a sunny Larnaca airport where luggage retrieval was efficient, but there was a slight delay until we met our driver and luxury coach. The crossing from the Greek to the Turkish side was rather different this year, with checks for the first time on the Greek side. However, completing a visa form to enter the north was no longer required, so the whole process was rather quicker. A lovely cool drink awaited us at Bellapais Monastery Village Hotel, where the presence of several long- serving staff was as reassuring as ever, and we soon settled in to our delightful rooms. Even at dusk, the temperature was such as to permit our introductory meeting to take place outside the dining room next to the swimming pool, during which a Eurasian Scops Owl flew across, attracted by the calls of a second bird. The buffet dinner was hailed a big hit, and most of the group retired early after a fairly exhausting day. -

Weed Flora of Vineyard in Bosnia and Herzegovina
In: D. Marčić, M. Glavendekić, P. Nicot (Eds.) Proceedings of the 7th Congress on Plant Protection. Plant Protection Society of Serbia, IOBC-EPRS, IOBC-WPRS, Belgrade, 2015, pp. 307 - 310 WEED FLORA OF VINEYARD IN BOSNIA AND HERZEGOVINA Zlatan Kovačević, Biljana Kelečević and Siniša Mitrić University of Banja Luka, Faculty of Agriculture Bulevar vojvode Petra Bojovica 1 A, 78000 Banja Luka, Bosnia and Herzegovina AbstrAct Two-year study (2008-2010) weed flora of vineyards in Bosnia and Herzegovina (B&H) performed on 51 locality. As result of this research it was found 133 species of vascular plants covered with: 112 genera, 39 families, 4 class and 2 divisions. The analysis of the biological spectrum showed 5 life forms with predominant presence of terophytes (45.86%), hemicryptophytes (39.85%) and geophytes (9.77%). Phytogeography analysis has been allocated 9 floristic groups, and the most common are: Cosmopolitan, Eurasian, Mediterranean, Boreal, Adventive and sub-Mediterranean, and together comprise 125 species (93.98%). It is very significant participation of 14 adventive species, and some species have taken invasive character, for example Ambrosia artemisiifolia L. Weed flora of vineyard in B&H is rich in flora due to the existence of continental and sub-Mediterranean wine-growing region. Considerable diversity is caused by the specifics of the study area, which are reflected in different climatic, edaphic and orographic characteristics, plant-geography, and different intensities of anthropogenic influences, traditions and the cultivation of grapevine. On the other hand it is important a presence of cosmopolitan and adventive species that are more or less extensively spread, and beside of typical weed and weed-ruderal species in weed flora of vineyards in B&H it was determined a significant number of ruderal and meadow species. -

Flora of South Australia 5Th Edition | Edited by Jürgen Kellermann
Flora of South Australia 5th Edition | Edited by Jürgen Kellermann PAPAVERACEAE (partly)1 Neville G. Walsh2 (subfam. Fumarioideae) & Jürgen Kellermann3 (family description) Herbaceous annuals or perennials, sometimes becoming shrubby as the inflorescence develops; most parts of the plant produce latex and contain alkaloids, leaves entire and often deeply dissected, pinnately or palmately compound, exstipulate. Inflorescence cymose or racemose, often a thyrse with leaf-like or membranous bracts; flowers bisexual, sepals 2 or 3, caducous; petals 4 or 6; stamens 4, 6 or numerous; ovary superior, carpels 2 or 3 or numerous (not in S.A.); in FUMARIOIDEAE : flowers either almost regular with petals in 2 whorls of differently shaped petals, stamens 4 (Hypecoum only), or flowers zygomorphic with sepals 2, in a lateral position, usually the same colour and texture as the corolla, and petals 4, in two whorls, with the 2 lateral ones being the inner ones, with the large dorsal one pouched or spurred at the base (with nectary scale), stamens 6, joined in an anterior and a posterior bundle, each consisting of 3 stamens, ovary surmounted by a style with a terminal 2- or 3-fid stigma, carpels 2, ovule 1 or more (outside S.A.); in PA P AVEROIDEAE : flowers regular, stamens numerous, ovary unilocular with numerous ovules; flowers regular, sepals caducous, stamens 4, ovary unilocular with numerous ovules. Fruit a capsule opening by valves or pores; seeds with small embryo, endosperm mealy or oily. Poppies, fumitories. The family is distributed throughout the temperate N hemisphere with some species in E Africa and S America; often grow in open areas or disturbed sites. -

On the Flora of Australia
L'IBRARY'OF THE GRAY HERBARIUM HARVARD UNIVERSITY. BOUGHT. THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEING AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. r^/f'ORElGN&ENGLISH' <^ . 1859. i^\BOOKSELLERS^.- PR 2G 1.912 Gray Herbarium Harvard University ON THE FLORA OF AUSTRALIA ITS ORIGIN, AFFINITIES, AND DISTRIBUTION. I I / ON THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEIKG AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. Reprinted from the JJotany of the Antarctic Expedition, Part III., Flora of Tasmania, Vol. I. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. 1859. PRINTED BY JOHN EDWARD TAYLOR, LITTLE QUEEN STREET, LINCOLN'S INN FIELDS. CONTENTS OF THE INTRODUCTORY ESSAY. § i. Preliminary Remarks. PAGE Sources of Information, published and unpublished, materials, collections, etc i Object of arranging them to discuss the Origin, Peculiarities, and Distribution of the Vegetation of Australia, and to regard them in relation to the views of Darwin and others, on the Creation of Species .... iii^ § 2. On the General Phenomena of Variation in the Vegetable Kingdom. All plants more or less variable ; rate, extent, and nature of variability ; differences of amount and degree in different natural groups of plants v Parallelism of features of variability in different groups of individuals (varieties, species, genera, etc.), and in wild and cultivated plants vii Variation a centrifugal force ; the tendency in the progeny of varieties being to depart further from their original types, not to revert to them viii Effects of cross-impregnation and hybridization ultimately favourable to permanence of specific character x Darwin's Theory of Natural Selection ; — its effects on variable organisms under varying conditions is to give a temporary stability to races, species, genera, etc xi § 3. -

The History of Phytotherapy and Pharmacognosy
HISTORY OF PHYTOTHERAPY AND PHARMACOGNOSY (compiled by Milan Nagy) PREHISTORIC EVIDENCE Where, in the eons of human evolution do we begin to chronicle the history of herba- lism? Archaeological studies at Shanidar in Iraq have shown that the eight species of pollen grains found at the burial site reveal that seven of these were plants that are still commonly used as folk medicine throughout the world. These include yarrow (Achillea), marshmallow (Althaea), groundsel (Senecio), centaury (Centaurea), ephedra and muscari. There are no de- finite reasons why Neanderthal man (ca. 60.000 B.C.) included these plants in their burial rites, but it is suggested that they were intended to "exert a beneficial effect on every impor- tant part of the body, and may have been chosen to fortify the dead man in his journey to ano- ther world." NEW STONE AGE (8,000 TO 5,000 B.C.) Transition from the paleolithic to neolithic period - from a food gathering to a food producing economy. Stone was polished, creating tools to clear trees, help farming. Lake- dwellers cultivated or gathered over two hundred different plants, among which are not a few that possess medicinal qualities: Papaver somniferum, Sambucus ebulus, Fumaria officinalis, Verbena officinalis, Saponaria officinalis, Menyanthes trifoliata., etc.. In the history of phytotherapy (herbalism), women prepared food and healing potions - women generally practiced herbalism on a day to day basis, as well as took care of the ills of other members of the family or tribal unit. However, throughout history, men compiled the remedies and wrote them down, which is why nearly all the herbals are by men. -

Department of Environment, Water and Natural Resources
Photograph: Helen Owens © Department of Environment, Water and Natural Resources, Government of South Australia Department of All rights reserved Environment, Copyright of illustrations might reside with other institutions or Water and individuals. Please enquire for details. Natural Resources Contact: Dr Jürgen Kellermann Editor, Flora of South Australia (ed. 5) State Herbarium of South Australia PO Box 2732 Kent Town SA 5071 Australia email: [email protected] Flora of South Australia 5th Edition | Edited by Jürgen Kellermann PAPAVERACEAE (partly)1 Neville G. Walsh2 (subfam. Fumarioideae) & Jürgen Kellermann3 (family description) Herbaceous annuals or perennials, sometimes becoming shrubby as the inflorescence develops; most parts of the plant produce latex and contain alkaloids, leaves entire and often deeply dissected, pinnately or palmately compound, exstipulate. Inflorescence cymose or racemose, often a thyrse with leaf-like or membranous bracts; flowers bisexual, sepals 2 or 3, caducous; petals 4 or 6; stamens 4, 6 or numerous; ovary superior, carpels 2 or 3 or numerous (not in S.A.); in FUMARIOIDEAE : flowers either almost regular with petals in 2 whorls of differently shaped petals, stamens 4 (Hypecoum only), or flowers zygomorphic with sepals 2, in a lateral position, usually the same colour and texture as the corolla, and petals 4, in two whorls, with the 2 lateral ones being the inner ones, with the large dorsal one pouched or spurred at the base (with nectary scale), stamens 6, joined in an anterior and a posterior bundle, each consisting of 3 stamens, ovary surmounted by a style with a terminal 2- or 3-fid stigma, carpels 2, ovule 1 or more; in PA P AVEROIDEAE : flowers regular, stamens numerous, ovary unilocular with numerous ovules; flowers regular, sepals caducous, stamens 4, ovary unilocular with numerous ovules. -

Fumitory (Fumaria Officinalis L.)1
Weed Technology. 1997. Volume 11:843-845 Intriguing World of Weeds iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii Fumitory (Fumaria officinalis L.) 1 LARRY W. MITICH2 INTRODUCTION AND ETYMOLOGY ing fumitory in 1670 (Simpson and Weiner 1989). The Fumitory (Fumaria officinalis L.) is a member of Fu specific epithet officinalis is from the Latin and means mariaceae, a family of annual and perennial herbs, "of shops," "sold in shops," or "official medicine" whose best-known genera, in addition to Fumaria, are (Gledhill 1985). Corydalis and Dicentra (bleeding heart and Dutchman's From the time of the Anglo-Saxons onwards, some breeches). Fumariaceae is mainly a species of Fumaria, particularly fumitory, became much temperate family that embraces 16 associated with witchcraft and superstition. The leaves genera and 400 species. Economi were burned for their smoke, which was firmly believed cally, its use is limited to garden to possess the power to expel and protect against evil ornamentals (Heywood 1993). spirits and spells (Allan 1978; Le Strange 1977). Brummitt (1992) and Hyam and About 55 species of Fumaria are known, the lll~ority Pankhurst (1995) place fumitory in of which are rather floppy, delicate, hairless annua~ with Papaveraceae, but most authorities, finely divided leaves and small, tubular, red to pink or including Mabberley (1989), put it whitish flowers. it is native mainly to Europe, including in Fumariaceae. the British Isles, Central Asia, and the Himalayas; how In 1753, Linnaeus established the genus Fumaria in ever, one species is tropical, native to the East African his Species Plantarum. He derived the name from the highlands (Hyam and Pankhurst 1995; Le Strange 1977). -

Beneficial Insects in Furrowed Field Margins
Beneficial insects in furrowed field margins Summary of a project by visiting student Benjamin Lepers, AgroParisTech France, Summer 2015 Agroecology group, James Hutton Institute, DS2 5DA, Dundee, UK In summer 2015, the Hutton hosted a visit by undergraduate student Benjamin Lepers from AgroParisTech. He was here to learn about vegetation and insect life in farmland. His first task was to complete a survey of plant species in different types of vegetation on the Mylnefield Farm, including land under cereal crops, grassy field margins, furrowed margins, taller mixed vegetation and hedges. The furrowed margins supported the largest number of plant species: 35-40 could be found in a brief survey. The furrowed margins – an idea developed on this farm – are called ‘Magic Margins’ and recently won awards for conservation and biodiversity (see News on the Hutton/LEAF web site). Sampling vegetation and insects on the furrowed margins The next aim was to examine recently formed furrowed margins to see how the vegetation varied on a small scale (e.g. over a few metres) and whether the proportions of plant species influenced the insects that live there. The margins supported different patches of vegetation, from 0.5 x 0.5 m up to 2 x 2 m, that arose naturally from the soil seedbank after furrowing. Three types were chosen for study: patches consisting of only grass species, those consisting of only fumitory species, mostly Fumaria officinalis and Fumaria muralis, and those of mixed broadleaf or dicotyledonous species which included Viola arvensis, Myosotis arvensis and Lamium amplexicaule. (The fumitories were the most common plant in these margins and tended to form dense clumps that excluded other broadleaf species). -

(Fumaria Parviflora Lam) (Fineleaf Fumitory) Grown in Ahwaz, Iran
Asian Journal of Chemistry Vol. 19, No. 7 (2007), 5336-5340 Chemical Composition of the Essential Oil of Fumitory Plant (Fumaria parviflora Lam) (Fineleaf fumitory) Grown in Ahwaz, Iran A. ASHNAGAR*, N. GHARIB NASERI† and M. YAGHOBI School of Pharmacy, Ahwaz Jundi Shapour University of Medical Sciences, Ahwaz, Iran E-mail: [email protected] Hydrodistillation of 100 g of powdered Fumitory parviflora Lam plant grown in the city of Ahwaz, Iran, resulted in the forma- tion of 1.2 mL of an essential oil. Gas chromatography-mass spec- trometry analysis of the volatile oil was carried on a DB-1 capil- lary column. On the basis of the results obtained it was concluded that the essential oil is consisted of 8 major components (72.7 %), 11 minor components (19.9 %) and 10 components with smaller amounts (4.5 %). Key Words: Fumitory, Fumitory parviflora, Fineleaf fumitory, Essential oil, GC-MS analysis. INTRODUCTION Fumaria is a genus of annual herbaceous flowering plants in the fam- ily Fumariaceae, native to temperate Europe and Asia. It is closely allied to Corydalis and some botanists combine the two genera. The common name is fumitory1. There are various species of this plant; some of the most important ones are listed in Fig. 12. Fumitory takes its name from the plant's blue-green colour, which is reminiscent of smoke rising from the earth. The name fumitory and the scientific name Fumaria derive from the Latin fumus terrae, meaning earth smoke. This is believed to stem from an early botanist who described the appearance of fumitory"as if the ground were all of a smoke3. -

Constituents and Pharmacology of Fumaria Officinalis- a Review
IOSR Journal Of Pharmacy (e)-ISSN: 2250-3013, (p)-ISSN: 2319-4219 Volume 10, Issue 1 Series. I (January 2020), PP. 17-25 www.iosrphr.org Constituents and Pharmacology of Fumaria Officinalis- A Review Ali Esmail Al-Snafi Department of Pharmacology, College of Medicine, Thi qar University, Iraq. Received 14 January 2020; Accepted 30 January 2020 Abstract: Fumaria officinalis contained alkaloids, carbohydrates, phenolic compounds, flavonoids, glycosides, terpenoids, phytosterols, proteins, amino acids, saponins, fixed oils, steroids, tannins and many other chemical constituents. The previous pharmacological studies showed that Fumaria officinalis possessed neural, analgesic, antioxidant, anticancer, antibacterial, antidiabetic, aphrodisiac effect and beneficial effect in biliary disorders and irritable bowel syndrome. This review highlighted the chemical constituents and pharmacological effects of Fumaria officinalis. Keywords: chemical constituents, pharmacology, Fumaria officinalis I. INTRODUCTION: As a result of accumulated experience from the past generations, today, all the world’s cultures have an extensive knowledge of herbal medicine. Two thirds of the new chemicals identified yearly were extracted from higher plants. 75% of the world’s population used plants for therapy and prevention. In the US, where chemical synthesis dominates the pharmaceutical industry, 25% of the pharmaceuticals are based on plant-derived chemicals (1). Plants are a valuable source of a wide range of secondary metabolites, which are used as pharmaceuticals, -

Ethnobotanical Study of the Sannio Area, Campania, Southern Italy
Ethnobotanical Study of the Sannio Area, Campania, Southern Italy Carmine Guarino, Luciana De Simone and Simona Santoro Research Abstract Ethnobotanical study of the Sannio area, in the province In the past medicinal plants were the first defence against of Benevento (SE-Italy) has been carried out on the basis diseases and they represented the only available thera- of both bibliographic sources and interviews in the field. In peutic means until chemistry started using nature as a total 365 species in 59 families were identified. Of these model for the synthesis of therapeutically active mole- species, 361 are medicinal plants, 82 for anthropic use cules. Nowadays, medicinal plants are still, in part, reso- lutive remedies, but, above all, they represent the first and 35 for veterinary use. source of active principles for medicaments as well as an essential source of molecular material for hemi-synthe- Riassunto sis processes (Calixto 2000, Cragg et al. 1997, De Smet 1997, Shu 1998). Uno studio etnobotanico del Sannio, in provincia di Be- nevento (SE-Italy), è stato effettuato sia sulla base di fon- Therefore, the interest in medicinal plants has remark- ti bibliografiche che di interviste in campo. In totale, 365 ably increased lately. In fact, a lot of works about vege- specie di 59 famiglie sono state censite. Di queste specie, table chemical structures and pharmaceutical properties 265 sono piante medicinali, 82 per uso antropico e 35 per have appeared. These works not only aim at discover- uso veterinario. ing new usable essences, but also at testing – by means of modern techniques - the properties and the effects of many plants now fallen into disuse or employed by popu- Introduction lar medicine in the past.