Natural Product Biosyntheses in Cyanobacteria: a Treasure Trove of Unique Enzymes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Subject Index
Cambridge University Press 0521462924 - Amino Acids and Peptides G. C. Barrett and D. T. Elmore Index More information Subject index acetamidomalonate synthesis 123–4 in fossil dating 15 S-adenosyl--methionine 11, 12, 174, 181 in geological samples 15 alanine, N-acetyl 9 in Nature 1 structure 4 IR spectrometry 36 alkaloids, biosynthesis from amino acids 16–17 isolation from proteins 121 alloisoleucine 6 mass spectra 61 allosteric change 178 metabolism, products of 187 allothreonine 6 metal-binding properties 34 Alzheimer’s disease 14 NMR 41 amidation at peptide C-terminus 57, 94, 181 physicochemical properties 32 amides, cis–trans isomerism 20 protein 3 N-acylation 72 PTC derivatives 87 N-alkylation 72 quaternary ammonium salts 50 hydrolysis 57 reactions of amino group 49, 51 O-trimethylsilylation 72 reactions of carboxy group 49, 53 reduction to -aminoalkanols 72 racemisation 56 amidocarbonylation in amino acid synthesis 125 routine spectrometry 35 amino acids, abbreviated names 7 Schöllkopf synthesis 127–8 acid–base properties 32 Schiff base formation 49 antimetabolites 200 sequence determination 97 et seq. as food additives 14 following selective chemical degradation 107 as neurotransmitters 17 following selective enzymic degradation 109 asymmetric synthesis 127 general strategy for 92 - 17–18 identification of C-terminus 106–7 biosynthesis 121 identification of N-terminus 94, 97 biosynthesis from, of creatinine 183 by solid-phase methodology 100 of nitric oxide 186 by stepwise chemical degradation 97 of porphyrins 185 by use of dipeptidyl -

Small Organic Molecules As Tunable Tools for Biology
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2015 Small Organic Molecules as Tunable Tools for Biology Unzue Lopez, Andrea Abstract: Drug discovery and development is a very challenging interdisciplinary endeavor that needs the contribution of medical doctors, biologists, chemists, X-ray crystallographers, and computer scientists, among many others, in order to be successful. The first part of this Ph. D. thesis focuses onthe development of EphB4 receptor tyrosine kinase inhibitors. EphB4 has been linked to angiogenesis, which involves the formation of new blood vessels supplying tumor cells with the necessary nutrients. Protein kinases play a key role in cell signaling by phosphorylating specific proteins and thus, the inhibition of their enzymatic activity by small organic molecules has been widely explored in drug design. In this work, the biological properties of an EphB4 inhibitor identified by computer simulations were improved bythe synthesis of several analogues. Their binding affinities were characterized by an array of biochemical and cell based assays, concluding with the validation of one of the most promising derivatives in an in vivo cancer xenograft model. The second part of the thesis deals with the development of novel bromodomain ligands starting from a micromolar potent in silico discovered hit. Bromodomain proteins are epigenetic readers that constitute an emerging topic in the field of drug discovery and are thus considered asvery attractive targets for the development of novel therapeutic drugs. A careful, structure-based design of analogues resulted in the discovery of nanomolar potent CREBBP ligands with an unprecedented selectivity profile among the bromodomain protein family. -

Cyanobacterial Bioactive Compounds: Biosynthesis, Evolution, Structure and Bioactivity
Cyanobacterial bioactive compounds: biosynthesis, evolution, structure and bioactivity Tânia Keiko Shishido Joutsen Division of Microbiology and Biotechnology Department of Food and Environmental Sciences Faculty of Agriculture and Forestry University of Helsinki, Finland and Integrative Life Science Doctoral Program University of Helsinki, Finland ACADEMIC DISSERTATION To be presented, with permission of the Faculty of Agriculture and Forestry of the University of Helsinki, for public examination in auditorium B2 (A109), at Viikki B- Building, Latokartanonkaari 7, on May 22nd 2015, at 12 o’clock noon. Helsinki 2015 1 Supervisors Professor Kaarina Sivonen Department of Food and Environmental Sciences University of Helsinki, Finland Docent David P. Fewer Department of Food and Environmental Sciences University of Helsinki, Finland Docent Jouni Jokela Department of Food and Environmental Sciences University of Helsinki, Finland Reviewers Professor Elke Dittmann Institute of Biochemistry and Biology University of Potsdam, Germany Professor Pia Vuorela Pharmaceutical Biology, Faculty of Pharmacy University of Helsinki, Finland Thesis committee Professor Mirja Salkinoja-Salonen Department of Food and Environmental Sciences University of Helsinki, Finland Docent Päivi Tammela Centre for drug research, Faculty of Pharmacy University of Helsinki, Finland Opponent Professor Vitor Vasconcelos Interdisciplinary Centre of Marine and Environmental Research University of Porto, Portugal Custos Professor Kaarina Sivonen Department of Food and Environmental -

Designing Peptidomimetics
CORE Metadata, citation and similar papers at core.ac.uk Provided by UPCommons. Portal del coneixement obert de la UPC DESIGNING PEPTIDOMIMETICS Juan J. Perez Dept. of Chemical Engineering ETS d’Enginyeria Industrial Av. Diagonal, 647 08028 Barcelona, Spain 1 Abstract The concept of a peptidomimetic was coined about forty years ago. Since then, an enormous effort and interest has been devoted to mimic the properties of peptides with small molecules or pseudopeptides. The present report aims to review different approaches described in the past to succeed in this goal. Basically, there are two different approaches to design peptidomimetics: a medicinal chemistry approach, where parts of the peptide are successively replaced by non-peptide moieties until getting a non-peptide molecule and a biophysical approach, where a hypothesis of the bioactive form of the peptide is sketched and peptidomimetics are designed based on hanging the appropriate chemical moieties on diverse scaffolds. Although both approaches have been used in the past, the former has been more widely used to design peptidomimetics of secretory peptides, whereas the latter is nowadays getting momentum with the recent interest in designing protein-protein interaction inhibitors. The present report summarizes the relevance of the information gathered from structure-activity studies, together with a short review on the strategies used to design new peptide analogs and surrogates. In a following section there is a short discussion on the characterization of the bioactive conformation of a peptide, to continue describing the process of designing conformationally constrained analogs producing first and second generation peptidomimetics. Finally, there is a section devoted to review the use of organic scaffolds to design peptidomimetics based on the information available on the bioactive conformation of the peptide. -

Algal Toxic Compounds and Their Aeroterrestrial, Airborne and Other Extremophilic Producers with Attention to Soil and Plant Contamination: a Review
toxins Review Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review Georg G¨аrtner 1, Maya Stoyneva-G¨аrtner 2 and Blagoy Uzunov 2,* 1 Institut für Botanik der Universität Innsbruck, Sternwartestrasse 15, 6020 Innsbruck, Austria; [email protected] 2 Department of Botany, Faculty of Biology, Sofia University “St. Kliment Ohridski”, 8 blvd. Dragan Tsankov, 1164 Sofia, Bulgaria; mstoyneva@uni-sofia.bg * Correspondence: buzunov@uni-sofia.bg Abstract: The review summarizes the available knowledge on toxins and their producers from rather disparate algal assemblages of aeroterrestrial, airborne and other versatile extreme environments (hot springs, deserts, ice, snow, caves, etc.) and on phycotoxins as contaminants of emergent concern in soil and plants. There is a growing body of evidence that algal toxins and their producers occur in all general types of extreme habitats, and cyanobacteria/cyanoprokaryotes dominate in most of them. Altogether, 55 toxigenic algal genera (47 cyanoprokaryotes) were enlisted, and our analysis showed that besides the “standard” toxins, routinely known from different waterbodies (microcystins, nodularins, anatoxins, saxitoxins, cylindrospermopsins, BMAA, etc.), they can produce some specific toxic compounds. Whether the toxic biomolecules are related with the harsh conditions on which algae have to thrive and what is their functional role may be answered by future studies. Therefore, we outline the gaps in knowledge and provide ideas for further research, considering, from one side, Citation: G¨аrtner, G.; the health risk from phycotoxins on the background of the global warming and eutrophication and, ¨а Stoyneva-G rtner, M.; Uzunov, B. -

Investigations on the Impact of Toxic Cyanobacteria on Fish : As
INVESTIGATIONS ON THE IMPACT OF TOXIC CYANOBACTERIA ON FISH - AS EXEMPLIFIED BY THE COREGONIDS IN LAKE AMMERSEE - DISSERTATION Zur Erlangung des akademischen Grades des Doktors der Naturwissenschaften an der Universität Konstanz Fachbereich Biologie Vorgelegt von BERNHARD ERNST Tag der mündlichen Prüfung: 05. Nov. 2008 Referent: Prof. Dr. Daniel Dietrich Referent: Prof. Dr. Karl-Otto Rothhaupt Referent: Prof. Dr. Alexander Bürkle 2 »Erst seit gestern und nur für einen Tag auf diesem Planeten weilend, können wir nur hoffen, einen Blick auf das Wissen zu erhaschen, das wir vermutlich nie erlangen werden« Horace-Bénédict de Saussure (1740-1799) Pionier der modernen Alpenforschung & Wegbereiter des Alpinismus 3 ZUSAMMENFASSUNG Giftige Cyanobakterien beeinträchtigen Organismen verschiedenster Entwicklungsstufen und trophischer Ebenen. Besonders bedroht sind aquatische Organismen, weil sie von Cyanobakterien sehr vielfältig beeinflussbar sind und ihnen zudem oft nur sehr begrenzt ausweichen können. Zu den toxinreichsten Cyanobakterien gehören Arten der Gattung Planktothrix. Hierzu zählt auch die Burgunderblutalge Planktothrix rubescens, eine Cyanobakterienart die über die letzten Jahrzehnte im Besonderen in den Seen der Voralpenregionen zunehmend an Bedeutung gewonnen hat. An einigen dieser Voralpenseen treten seit dem Erstarken von P. rubescens existenzielle, fischereiwirtschaftliche Probleme auf, die wesentlich auf markante Wachstumseinbrüche bei den Coregonenbeständen (Coregonus sp.; i.e. Renken, Felchen, etc.) zurückzuführen sind. So auch -

Polyketide Synthase Gene Coupled to the Peptide Synthetase Module Involved in the Biosynthesis of the Cyclic Heptapeptide Microcystinl
J. Biochem. 127, 779-789(2000) Polyketide Synthase Gene Coupled to the Peptide Synthetase Module Involved in the Biosynthesis of the Cyclic Heptapeptide Microcystinl Tomoyasu Nishizawa, Akiko Ueda, Munehiko Asayama,* Kiyonaga Fujii,•õ Ken-ichi Harada,i Kozo Ochi,•ö and Makoto Shirai*,•˜,2 * Division of Biotechnology, School ofAgriculture, Ibaraki University Ami , Ibaraki 300-0393; •õFaculty of Pharmacy, M eija University, Tempaku , Nagoya 468-8503, •öNational Food Research Institute, Tsukuba, Ibaraki 305-8642; and •˜ Gene Research Center, Ibaraki University, Ann, Ibaraki 300-0393 Received January 17, 2000; accepted February 11, 2000 The peptide synthetase gene operon, which consists of encyA, mcyB, and mcyC, for the activation and incorporation of the five amino acid constituents of microcystin has been identified [T. Nishizawa et al. (1999) J. Biochem. 126, 520-529] . By sequencing an addi tional 34 kb of DNA from microcystin-producing Microcystis aeruginosa K-139 , we identifi ed the residual microcystin synthetase gene operon, which consists of mcyD, mcyE, meyF, and mcyG, in the opposite orientation to the mcyABC operon. McyD consisted of two polyketide synthase modules, and McyE contained a polyketide synthase module at the N-terminus and a peptide synthetase module at the C-terminus. McyF was found to exhibit similarity to amino acid racemase. McyG consisted of a peptide synthetase mod ule at the N-terminus and a polyketide synthase at the C-terminus. The microcystin syn thetase gene cluster was conserved in another microcystin-producing strain, Microcystis sp. S-70, which produces Microcystin-LR, -RR, and -YR. Insertional mutagenesis of mcyA, mcyD, or meyE in Microcystis sp. -

Interrupted Adenylation Domains: Unique Bifunctional Enzymes Involved in Nonribosomal Peptide Biosynthesis
Natural Product Reports Interrupted adenylation domains: unique bifunctional enzymes involved in nonribosomal peptide biosynthesis Journal: Natural Product Reports Manuscript ID: NP-REV-09-2014-000120.R1 Article Type: Highlight Date Submitted by the Author: 12-Jan-2015 Complete List of Authors: Labby, Kristin; Beloit College, Chemistry Watsula, Stoyan; University of Michigan, Medicinal Chemistry Garneau-Tsodikova, Sylvie; University of Kentucky, Pharmaceutical Sciences Page 1 of 11NPR Natural Product Reports Dynamic Article Links ► Cite this: DOI: 10.1039/c0xx00000x www.rsc.org/xxxxxx HIGHLIGHT Interrupted adenylation domains: unique bifunctional enzymes involved in nonribosomal peptide biosynthesis Kristin J. Labby, a Stoyan G. Watsula,b and Sylvie Garneau-Tsodikova* c Received (in XXX, XXX) Xth XXXXXXXXX 20XX, Accepted Xth XXXXXXXXX 20XX 5 DOI: 10.1039/b000000x Covering up to 2014 Nonribosomal peptides (NRPs) account for a large portion of drugs and drug leads currently available in the pharmaceutical industry. They are one of two main families of natural products biosynthesized on megaenzyme assembly-lines composed of multiple modules that are, in general, each comprised of three 10 core domains and on occasion of accompanying auxiliary domains. The core adenylation (A) domains are known to delineate the identity of the specific chemical components to be incorporated into the growing NRPs. Previously believed to be inactive, A domains interrupted by auxiliary enzymes have recently been proven to be active and capable of performing two distinct chemical reactions. This highlight summarizes current knowledge on A domains and presents the various interrupted A domains found in a number of 15 nonribosomal peptide synthetase (NRPS) assembly-lines, their predicted or proven dual functions, and their potential for manipulation and engineering for chemoenzymatic synthesis of new pharmaceutical agents with increased potency. -

FMB Ch05-Gerwick.Indd
PB Marine Cyanobacteria Ramaswamy et al. 175 5 The Secondary Metabolites and Biosynthetic Gene Clusters of Marine Cyanobacteria. Applications in Biotechnology Aishwarya V. Ramaswamy, Patricia M. Flatt, Daniel J. Edwards, T. Luke Simmons, Bingnan Han and William H. Gerwick* Abstract Marine cyanobacteria have proven to be one of the most versatile marine producers of secondary metabolites. Many of these metabolites demonstrate antiproliferative activity (e.g. curacin A, dolastatins), acute cytotoxic activity (e.g. apratoxin, hectochlorin) or have specific neurotoxic activity (e.g. kalkitoxin, antillatoxin), making them invaluable as potential therapeutic leads or pharmacological tools. The predominant biogenetic theme in cyanobacterial natural products chemistry is the integration of polyketide synthases (PKS) and nonribosomal peptide synthetases (NRPS) along with a variety of unusual tailoring or modifying enzymes, and accounts for the tremendous structural diversity of their metabolites. Only recently has the genetic architecture of several cyanobacterial biosynthetic gene clusters been determined, and studies to understand and exploit this biosynthentic machinery present an exciting new frontier. This chapter will summarize the properties of several notable metabolites from marine cyanobacteria that have clinical or pharmacological applications followed by a detailed account of their biosyntheses at the molecular genetic level and their potential applications in biotechnology. 1. Introduction Cyanobacteria, also known as blue-green algae, are ancient (ca. 2 x 109 years) photosynthetic prokaryotes which inhabit a wide diversity of habitats including *For correspondence email [email protected] 176 Marine Cyanobacteria Ramaswamy et al. 177 open oceans, tropical reefs, shallow water environments, terrestrial substrates, aerial environments such as in trees and rock faces, and fresh water ponds, streams and puddles (Whitton and Potts, 2000) . -

COMPILATION of AMINO ACIDS, DRUGS, METABOLITES and OTHER COMPOUNDS in MASSTRAK AMINO ACID ANALYSIS SOLUTION Paula Hong, Kendon S
COMPILATION OF AMINO ACIDS, DRUGS, METABOLITES AND OTHER COMPOUNDS IN MASSTRAK AMINO ACID ANALYSIS SOLUTION Paula Hong, Kendon S. Graham, Alexandre Paccou, T homas E. Wheat and Diane M. Diehl INTRODUCTION LC conditions Physiological amino acid analysis is commonly performed to LC System: Waters ACQUITY UPLC® System with TUV monitor and study a wide variety of metabolic processes. A wide Column: MassTrak AAA Column 2.1 x 150 mm, 1.7 µm variety of drugs, foods, and metabolic intermediates that may Column Temp: 43 ˚C be present in biological fluids can appear as peaks in amino Flow Rate: 400 µL/min. acid analysis, therefore, it is important to be able to identify Mobile Phase A: MassTrak AAA Eluent A Concentrate, unknown compounds.1,2,3 The reproducibility and robustness of diluted 1:10 the MassTrak Amino Acid Analysis Solution make this method well Mobile Phase B: MassTrak AAA Eluent B suited to such a study as well.4 Weak Needle Wash: 5/95 Acetonitrile/Water Strong Needle Wash: 95/5 Acetonitrile/Water Gradient: MassTrak AAA Standard Gradient (as provided in kit) Detection: UV @ 260 nm Injection Volume: 1 µL EXPERIMENTAL Injection Mode: Partial Loop with Needle Overfill (PLNO) Compound sample preparation A library of compounds was assembled. Each compound was derivatized individually and spiked into the MassTrak™ AAA Solution Standard prior to chromatographic analysis. The elution RESULTS AND DISCUSSION position of each tested compound could be related to known amino acids. A wide variety of antibiotics, pharmaceutical compounds and metabolite by-products are found in biological fluids. The reten- 1. -
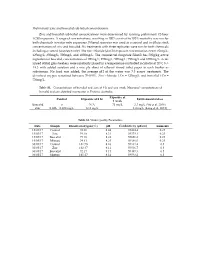
Supplementary File 1 (PDF, 650 Kib)
Preliminary Zinc and boscalid sub lethal concentrations Zinc and boscalid sub lethal concentrations were determined by running preliminary 72‐hour LC50 exposures. A range of concentrations, resulting in 100% survival to 100% mortality was run for both chemicals in water only exposures. Filtered seawater was used as a control and to dilute stock concentrations of zinc and boscalid. Six treatments with three replicates were run for both chemicals including a control (seawater only). The zinc chloride (ZnCl2) exposure concentrations were: 62mg/L; 125mg/L; 250mg/L; 500mg/L and 1000mg/L. The commercial fungicide Filan® has 500g/kg active ingredient of boscalid, concentrations of 100mg/L; 250mg/L; 500mg/L; 750mg/L and 1000mg/L. Acid‐ rinsed 600ml glass beakers were randomly placed in a temperature‐controlled incubator at 20oC (+/‐ 1oC) with added aeration and a one ply sheet of ethanol rinsed toilet paper in each beaker as substratum. No food was added; the average pH of the water was 7.5 across treatments. The dissolved oxygen remained between 70‐100%. Zinc chloride LC50 = 125mg/L and boscalid LC50 = 750mg/L. Table S1. –Concentrations of boscalid and zinc at 0 h and one week. Measured concentrations of boscalid and zinc detected in estuaries in Victoria, Australia. Exposure at Control Exposure at 0 hr Environmental dose 1 week Boscalid 0 N/A 75 mg/L 3.3 mg/L (Vu et al. 2016) Zinc 0.026 – 0.034 mg/L 12.5 mg/L 3.4 mg/L (Long et al. 2015) Table S2. Water Quality Parameters. Date Sample Dissolved oxygen (%) pH Conductivity (µS/cm) Ammonia 15/05/17 Control 98.36 8.44 59464.4 0.25 15/05/17 Zinc 99.10 8.31 59379.1 0.25 15/05/17 Boscalid 99.18 8.28 59656.8 0.25 15/05/17 Mixture 94.81 8.25 59386.0 0.25 30/05/17 Control 101.90 8.16 59332.4 0.5 30/05/17 Zinc 102.47 8.11 59536.7 0.5 30/05/17 Boscalid 92.17 8.15 59489.3 0.5 30/05/17 Mixture 101.57 8.14 59593.2 0.5 Supplementary Material . -

Multiple Toxin Production in the Cyanobacterium Microcystis: Isolation of the Toxic Protease Inhibitor Cyanopeptolin 1020
Gademann, K; Portmann, C; Blom, J F; Zeder, M; Jüttner, F (2010). Multiple toxin production in the cyanobacterium microcystis: isolation of the toxic protease inhibitor cyanopeptolin 1020. Journal of Natural Products, 73(5):980-984. Postprint available at: http://www.zora.uzh.ch University of Zurich Posted at the Zurich Open Repository and Archive, University of Zurich. Zurich Open Repository and Archive http://www.zora.uzh.ch Originally published at: Gademann, K; Portmann, C; Blom, J F; Zeder, M; Jüttner, F (2010). Multiple toxin production in the Winterthurerstr. 190 cyanobacterium microcystis: isolation of the toxic protease inhibitor cyanopeptolin 1020. Journal of Natural CH-8057 Zurich Products, 73(5):980-984. http://www.zora.uzh.ch Year: 2010 Multiple toxin production in the cyanobacterium microcystis: isolation of the toxic protease inhibitor cyanopeptolin 1020 Gademann, K; Portmann, C; Blom, J F; Zeder, M; Jüttner, F Gademann, K; Portmann, C; Blom, J F; Zeder, M; Jüttner, F (2010). Multiple toxin production in the cyanobacterium microcystis: isolation of the toxic protease inhibitor cyanopeptolin 1020. Journal of Natural Products, 73(5):980-984. Postprint available at: http://www.zora.uzh.ch Posted at the Zurich Open Repository and Archive, University of Zurich. http://www.zora.uzh.ch Originally published at: Gademann, K; Portmann, C; Blom, J F; Zeder, M; Jüttner, F (2010). Multiple toxin production in the cyanobacterium microcystis: isolation of the toxic protease inhibitor cyanopeptolin 1020. Journal of Natural Products, 73(5):980-984. Multiple toxin production in the cyanobacterium microcystis: isolation of the toxic protease inhibitor cyanopeptolin 1020 Abstract The isolation and structure of cyanopeptolin 1020 (hexanoic acid-Glu-N[-O-Thr-Arg-Ahp-Phe-N-Me-Tyr-Val-]) from a Microcystis strain is reported.