I KWAME NKRUMAH UNIVERSITY of SCIENCE and TECHNOLOGY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![The Nephrolepis Boston Fern Complex Series Editors (Including Nephrolepis Exaltata [L.] Dan Blanchon Schott), Nephrolepidaceae, Naturalised in New Zealand](https://docslib.b-cdn.net/cover/6282/the-nephrolepis-boston-fern-complex-series-editors-including-nephrolepis-exaltata-l-dan-blanchon-schott-nephrolepidaceae-naturalised-in-new-zealand-176282.webp)
The Nephrolepis Boston Fern Complex Series Editors (Including Nephrolepis Exaltata [L.] Dan Blanchon Schott), Nephrolepidaceae, Naturalised in New Zealand
PERSPECTIVES IN Biosecurity RESEARCH SERIES 2/2016 The Nephrolepis Boston fern complex SERIES EDITORS (including Nephrolepis exaltata [L.] Dan Blanchon Schott), Nephrolepidaceae, naturalised in New Zealand. Mel Galbraith Mark Large and Lizzy Farrington PERSPECTIVES IN BIOSECURITY RESEARCH SERIES 2/2016 The Nephrolepis Boston fern complex (including Nephrolepis exaltata [L.] Schott), Nephrolepidaceae, naturalised in New Zealand. By Mark Large and Lizzy Farrington The Nephrolepis Boston fern complex (including Nephrolepis exaltata [L.] Schott), Nephrolepidaceae, naturalised in New Zealand by Mark Large and Lizzy Farrington is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. This publication may be cited as: Large, M., and Farrington, L. (2016). The Nephrolepis Boston fern complex (including Nephrolepis exaltata [L.] Schott), Nephrolepidaceae, naturalised in New Zealand. Unitec ePress Perspectives in Biosecurity Research Series (2). Retrieved from http://www.unitec.ac.nz/epress/ About this series: Perspectives in Biosecurity is an occasional, multi-disciplinary electronic series of research papers and other outputs covering all aspects of the field of biosecurity, including, but not restricted to: invasion biology and ecology, invasive species identification/ diagnostics, management and eradication/control, new invasive species records, modelling, biosecurity law and policy, relationships between human society and invasive species. Papers in Perspectives in Biosecurity are primarily the results of research carried out by staff, students, graduates, associates, and collaborators of Unitec Institute of Technology. All papers are subject to a double blind peer review process. For more papers in this series please visit: www.unitec.ac.nz/epress/index.php/category/publications/epress-series/perspectives-in-biosecurity/ Cover design by Penny Thomson Cover image by Mel Galbraith On the cover is the Australian tachinid fly (Trigonospila brevifacies), a parasitoid of other insects, specifically larvae of a number of Lepidoptera. -

Survey of Ornamental Ferns, Their Morphology and Uses for Environmental Protection, Improvement and Management
Ife Journal of Science vol. 14, no. 2 (2012) 245 SURVEY OF ORNAMENTAL FERNS, THEIR MORPHOLOGY AND USES FOR ENVIRONMENTAL PROTECTION, IMPROVEMENT AND MANAGEMENT. Oloyede, F. A. Department of Botany, Obafemi Awolowo University, Ile-Ife. E-mail: [email protected] (Received: April 2012; Accepted: August 2012) ABSTRACT A survey of ornamental ferns with potentials for environmental protection, improvement and management was conducted at Obafemi Awolowo University, Ile-Ife, Nigeria and villages around it between 2008-2012. The collections were characterized morphologically. This was with a view to creating awareness of the importance of ornamental ferns for environmental management and economic values. Ferns were collected from humid forest, rainforest and re-growth forest vegetation zones, open bare land, around homes, offices and road sides. All the ferns collected were raised at the Department of Botany, OAU, Ile-Ife. Morphological, reproductive and growth parameters were observed, documented for their ornamental values and for their relevance in environmental protection, improvement and management. The result showed that there were a lot of variations in the shape and type of apex, frond length and diameters; leaflet texture, size and shapes in all the ferns studied. There were little variations in the structures of rhizome except in Phymatodes scholopendria with long-creeping, climbing, succulent rhizome. The only leaflet with petiole was found in Adiantum veneris-capillus. They have shoot and root biomass which are directly involved in checking wind and soil erosion by covering and holding soils together. These ornamental ferns were found to have much value for landscaping and aesthetic purposes but they were found in few villages with little or no attention. -

Plant Species List for Bob Janes Preserve
Plant Species List for Bob Janes Preserve Scientific and Common names obtained from Wunderlin 2013 Scientific Name Common Name Status EPPC FDA IRC FNAI Family: Azollaceae (mosquito fern) Azolla caroliniana mosquito fern native R Family: Blechnaceae (mid-sorus fern) Blechnum serrulatum swamp fern native Woodwardia virginica Virginia chain fern native R Family: Dennstaedtiaceae (cuplet fern) Pteridium aquilinum braken fern native Family: Nephrolepidaceae (sword fern) Nephrolepis cordifolia tuberous sword fern exotic II Nephrolepis exaltata wild Boston fern native Family: Ophioglossaceae (adder's-tongue) Ophioglossum palmatum hand fern native E I G4/S2 Family: Osmundaceae (royal fern) Osmunda cinnamomea cinnamon fern native CE R Osmunda regalis royal fern native CE R Family: Polypodiaceae (polypody) Campyloneurum phyllitidis long strap fern native Phlebodium aureum golden polypody native Pleopeltis polypodioides resurrection fern native Family: Psilotaceae (whisk-fern) Psilotum nudum whisk-fern native Family: Pteridaceae (brake fern) Acrostichum danaeifolium giant leather fern native Pteris vittata China ladder break exotic II Family: Salviniaceae (floating fern) Salvinia minima water spangles exotic I Family: Schizaeaceae (curly-grass) Lygodium japonicum Japanese climbing fern exotic I Lygodium microphyllum small-leaf climbing fern exotic I Family: Thelypteridaceae (marsh fern) Thelypteris interrupta hottentot fern native Thelypteris kunthii widespread maiden fern native Thelypteris palustris var. pubescens marsh fern native R Family: Vittariaceae -
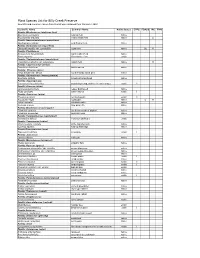
Plant Species List for Billy Creek Preserve Scientific and Common Names from This List Were Obtained from Wunderlin 2003
Plant Species List for Billy Creek Preserve Scientific and Common names from this list were obtained from Wunderlin 2003 Scientific Name Common Name Native Status EPPC FDACS IRC FNAI Family: Blechnaceae (midsorus fern) Blechnum serrulatum swamp fern native Woodwardia areolata netted chain fern native CI Family: Nephrolepidaceae (sword fern) Nephrolepis exaltata wild Boston fern native Family: Osmundaceae (royal fern) Osmunda regalis var. spectabilis royal fern native CE R Family: Pteridaceae Acrostichum danaeifolium giant leather fern native Pteris tripartita Giant brake fern exotic Family: Thelypteridaceae (marsh fern) Thelypteris palustris var. pubescens marsh fern native R Family: Cupressaceae (cedar) Taxodium distichum bald-cypress native Family: Pinaceae (pine) Pinus elliottii var. densa south Florida slash pine native Family: Alismataceae (water plantain) Sagittaria latifolia broadleaf arrowhead native Family: Asparagaceae Sansevieria hyacinthoides bowstring hemp, mother-in-law's tongue exotic II Family: Araceae (arum) Lemna aequinoctialis lesser duckweed native Pistia stratiotes water lettuce exotic I Family: Arecaceae (palm) Phoenix reclinata reclinata palm exotic II Roystonea regia royal palm native E R Sabal palmetto cabbage palm native Serenoa repens saw palmetto native Family: Bromeliaceae (pineapple) Tillandsia setacea southern needleaf airplant native Tillandsia usneoides spanish moss native Family: Commelinaceae (spiderwort) Commelina diffusa common dayflower exotic Family: Cyperaceae (sedge) Rhynchospora colorata white -

Lyonia Preserve Plant Checklist
I -1 Lyonia Preserve Plant Checklist Volusia County, Florida I, I Aceraceae (Maple) Asteraceae (Aster) Red Maple Acer rubrum • Bitterweed Helenium amarum • Blackroot Pterocaulon virgatum Agavaceae (Yucca) Blazing Star Liatris sp. B Adam's Needle Yucca filamentosa Blazing Star Liatris tenuifolia BNolina Nolina brittoniana Camphorweed Heterotheca subaxillaris Spanish Bayonet Yucca aloifolia § Cudweed Gnaphalium falcatum • Dog Fennel Eupatorium capillifolium Amaranthaceae (Amaranth) Dwarf Horseweed Conyza candensis B Cottonweed Froelichia floridana False Dandelion Pyrrhopappus carolinianus • Fireweed Erechtites hieracifolia B Anacardiaceae (Cashew) Garberia Garberia heterophylla Winged Sumac Rhus copallina Goldenaster Pityopsis graminifolia • § Goldenrod Solidago chapmanii Annonaceae (Custard Apple) Goldenrod Solidago fistulosa Flag Paw paw Asimina obovata Goldenrod Solidago spp. B • Mohr's Throughwort Eupatorium mohrii Apiaceae (Celery) BRa gweed Ambrosia artemisiifolia • Dollarweed Hydrocotyle sp. Saltbush Baccharis halimifolia BSpanish Needles Bidens alba Apocynaceae (Dogbane) Wild Lettuce Lactuca graminifolia Periwinkle Catharathus roseus • • Brassicaceae (Mustard) Aquifoliaceae (Holly) Poorman's Pepper Lepidium virginicum Gallberry Ilex glabra • Sand Holly Ilex ambigua Bromeliaceae (Airplant) § Scrub Holly Ilex opaca var. arenicola Ball Moss Tillandsia recurvata • Spanish Moss Tillandsia usneoides Arecaceae (Palm) • Saw Palmetto Serenoa repens Cactaceae (Cactus) BScrub Palmetto Sabal etonia • Prickly Pear Opuntia humifusa Asclepiadaceae -

Nothodactylaria Nephrolepidis Fungal Planet Description Sheets 297
296 Persoonia – Volume 43, 2019 Nothodactylaria nephrolepidis Fungal Planet description sheets 297 Fungal Planet 979 – 18 December 2019 Nothodactylariaceae Crous, fam. nov. Etymology. Name refers to the genus Nothodactylaria. integrated, hyaline to pale brown, smooth, subcylindrical with apical taper, forming a rachis with sympodially proliferating Classification — Nothodactylariaceae, Xylariales, Sordario- pimple-like denticles. Conidia solitary, aggregating in a mucoid mycetes. mass, septate, hyaline, smooth, subcylindrical to fusoid-ellip- Mycelium consisting of hyaline, smooth, branched, septate, soid, straight, apex obtuse, tapering to truncate hilum. hyphae. Conidiophores solitary or aggregated in clusters, sub- Type genus. Nothodactylaria Crous. cylindrical, unbranched, erect, hyaline to pale brown, smooth, MycoBank MB833022. with slight apical taper, septate. Conidiogenous cells terminal, Nothodactylaria Crous, gen. nov. Etymology. Name refers to its similarity with Dactylaria. apical taper, forming a rachis with sympodially proliferating pimple-like denticles. Conidia solitary, aggregating in a mucoid Mycelium consisting of hyaline, smooth, branched, septate, mass, septate, hyaline, smooth, subcylindrical to fusoid-ellip- hyphae. Conidiophores solitary or aggregated in clusters, sub- soid, straight, apex obtuse, tapering to truncate hilum. cylindrical, unbranched, erect, hyaline to pale brown, smooth, with slight apical taper, septate. Conidiogenous cells terminal, Type species. Nothodactylaria nephrolepidis Crous. integrated, hyaline to pale brown, smooth, subcylindrical with MycoBank MB833023. Nothodactylaria nephrolepidis Crous, sp. nov. Etymology. Name refers to the host genus Nephrolepis from which it was Notes — Dactylaria is characterised by having hyaline co- isolated. nidiophores and septate, hyaline conidia formed on denticles Mycelium consisting of hyaline, smooth, branched, septate, (De Hoog 1985). The genus Dactylaria is polyphyletic, and 1.5–2 µm diam hyphae. Conidiophores solitary or aggregated the phylogeny of its type species (D. -

ISB: Atlas of Florida Vascular Plants
Page 1 of 8 Spruce Creek Plant List Acanthaceae Annonaceae Firespike Odontonema cuspidatum Flag Pawpaw Asimina obovata Wild Petunia Ruellia caroliniensis Small Flower Pawpaw Asimina parviflora Whitelady Thunbergia fragrans Dwarf Pawpaw Asimina pygmea Woolly Pawpaw Asimina speciosa Adoxacease Pawpaw Asimina spp. Elderberry Sambucus canadensis Walter's Viburnum Viburnum obovatum Apiaceae Dollarweed Hydrocotyle sp. Agavaceae Mock Bishopweed Ptilimnium capillaceum Spanish Bayonet Yucca aloifolia Apocynaceae Aizoaceae Leafless Swallowwort Cynanchum scoparium Sea Purslane Sesuvium portulacastrum Swallowwort Cynanchum scp. Alismataceae Aquifoliaceae Duck Potato Sagittaria latifolia Sand Holly Ilex ambigua Arrowhead Sagittaria sagittifolia Dahoon Holly Ilex cassine Possum-Haw Ilex decidua Altingiaceae Gallberry Ilex glabra Sweetgum Liquidambar styraciflua American Holly llex opaca Scrub Holly Ilex opaca var. arenicola Amaranthaceae Yaupon Holly Ilex vomitoria Slim amaranth Amaranthus hybridus Glasswort Salicornia bigelovii Araceae Glasswort Salicornia virginica Amercian Evergreen Syngonium podophgyllum Perrennial Glasswort Sarcocornia ambigua Sea Blite Suaeda linearis Araliaceae Asian Coin Wort Centella asiatica Amaryllidaceae Swamplily Crinum americanum Arecaceae Scrub Palmetto Sal palmetto Anacardiaceae Cabbage Palm Sabal palmetto Winged Sumac Rhus copallinum Saw Palmetto Serenoa repens Brazilian Pepper Schinus terebinthifolia Poison Ivy Toxicodendron radicans Arthoniaceae Red Blanket Lichen Herpothallon rubrocinctum Page 2 of 8 Asparagaceae -

Houseplants Have an Almost Magical Ability to Transform an Otherwise Drab and Dreary House Into an Aesthetically Pleasing, Peaceful Home
During the winter months, surround yourself with houseplants and breathe a little easier! Houseplants have an almost magical ability to transform an otherwise drab and dreary house into an aesthetically pleasing, peaceful home. But they can also improve indoor air quality. Beautiful houseplants serve as an additional air filtration system in your home. Peggy Anderson, Greenhouse Associate at Countryside Flower Shop and Nursery in Crystal Lake, recommends these plants as the most effective air cleaners: Peace Lily (Spathiphyllum wallisii) – The Peace Lily is a favorite to many customers especially when it blooms. This graceful flowering plant thrives in light to moderate shade. It tops the list in air-purifying plants removing benzene, formaldehyde, trichloroethylene, xylene, toluene and ammonia from the air you breathe indoors. Pothos (Epipremnum aureum) – Great for the beginner or someone who may not be confident in their plant care. This super easy to care for plant enjoys bright indirect light. Pothos also remove the above-mentioned toxins from your indoor air. Mother-in-Law’s Tongue (Sanseviera trifasciata) – These stiff, upright leaves reach up to 4 feet tall and come with stripes of different variegations. It will withstand a variety of indoor conditions and prefers a dry soil. English Ivy (Hedera helix) – Although a little trickier to care for, Ivy plants lend themselves nicely to topiaries, used as a trailing plant or as a climber with support. English Ivy has a natural ability to filter pollutants from your indoor air as well. Weeping Fig (Ficus benjamina) – Weeping Figs grow beautifully in a brightly lit room, preferring direct sunlight. -

Phylogenetic Analyses Place the Monotypic Dryopolystichum Within Lomariopsidaceae
A peer-reviewed open-access journal PhytoKeysPhylogenetic 78: 83–107 (2017) analyses place the monotypic Dryopolystichum within Lomariopsidaceae 83 doi: 10.3897/phytokeys.78.12040 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research Phylogenetic analyses place the monotypic Dryopolystichum within Lomariopsidaceae Cheng-Wei Chen1,*, Michael Sundue2,*, Li-Yaung Kuo3, Wei-Chih Teng4, Yao-Moan Huang1 1 Division of Silviculture, Taiwan Forestry Research Institute, 53 Nan-Hai Rd., Taipei 100, Taiwan 2 The Pringle Herbarium, Department of Plant Biology, The University of Vermont, 27 Colchester Ave., Burlington, VT 05405, USA 3 Institute of Ecology and Evolutionary Biology, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, 10617, Taiwan 4 Natural photographer, 664, Hu-Shan Rd., Caotun Township, Nantou 54265, Taiwan Corresponding author: Yao-Moan Huang ([email protected]) Academic editor: T. Almeida | Received 1 February 2017 | Accepted 23 March 2017 | Published 7 April 2017 Citation: Chen C-W, Sundue M, Kuo L-Y, Teng W-C, Huang Y-M (2017) Phylogenetic analyses place the monotypic Dryopolystichum within Lomariopsidaceae. PhytoKeys 78: 83–107. https://doi.org/10.3897/phytokeys.78.12040 Abstract The monotypic fern genusDryopolystichum Copel. combines a unique assortment of characters that ob- scures its relationship to other ferns. Its thin-walled sporangium with a vertical and interrupted annulus, round sorus with peltate indusium, and petiole with several vascular bundles place it in suborder Poly- podiineae, but more precise placement has eluded previous authors. Here we investigate its phylogenetic position using three plastid DNA markers, rbcL, rps4-trnS, and trnL-F, and a broad sampling of Polypodi- ineae. -

Current State of Indoor Air Phytoremediation Using Potted Plants and Green Walls
atmosphere Review Current State of Indoor Air Phytoremediation Using Potted Plants and Green Walls Samaneh Bandehali 1, Taghi Miri 2,* , Helen Onyeaka 2 and Prashant Kumar 3 1 Department of Chemical Engineering, Arak University, Arak 38156-8-8349, Iran; [email protected] 2 School of Chemical Engineering, University of Birmingham, Birmingham B15 2TT, UK; [email protected] 3 Global Centre for Clean Air Research (GCARE), Department of Civil and Environmental Engineering, Faculty of Engineering and Physical Sciences, University of Surrey, Guildford GU2 7XH, UK; [email protected] * Correspondence: [email protected] Abstract: Urban civilization has a high impact on the environment and human health. The pollution level of indoor air can be 2–5 times higher than the outdoor air pollution, and sometimes it reaches up to 100 times or more in natural/mechanical ventilated buildings. Even though people spend about 90% of their time indoors, the importance of indoor air quality is less noticed. Indoor air pollution can be treated with techniques such as chemical purification, ventilation, isolation, and removing pollutions by plants (phytoremediation). Among these techniques, phytoremediation is not given proper attention and, therefore, is the focus of our review paper. Phytoremediation is an affordable and more environmentally friendly means to purify polluted indoor air. Furthermore, studies show that indoor plants can be used to regulate building temperature, decrease noise levels, and alleviate social stress. Sources of indoor air pollutants and their impact on human health are briefly discussed in this paper. The available literature on phytoremediation, including experimental works for removing volatile organic compound (VOC) and particulate matter from the indoor air and associated challenges and opportunities, are reviewed. -

Notes on the Palmetto Weevil, Rhynchophoru5 Cruentatus (Coleoptera: Curculionidae)1
The cyclical nature of the color response was probably needed under Florida conditions to identify the level of due to the leaching and uptake of nitrogen, denitrification nitrogen in the soil solution which corresponds to moder or volatilization of ammonia (NH3) which is associated with ate plant growth, acceptable color and little or no leaching soil urea application (3). The magnitude of the color de into the ground water. Relate work being conducted at a cline 4 weeks after treatment was reduced at the higher number of institutions for nursery crops grown in contain nitrogen rates; however, fertilizer cost increases, excessive ers needs to be expanded to include the affects of fertiliza shoot growth may result and turf may be injured when tion on plant growth, leaching and runoff on landscape applying more than 1 lb N/1000 ft2 in one application in sites. hot weather (2). This could become important if the fer tilized plant was growing in a turf area and the material Literature Cited was surface broadcast applied over the turf. If more than 1 lb N/1000 ft2 were to be applied to a turf area, consider 1. Dasberg, S. 1987. Nitrogen fertilization in citrus orchards. Plant and ation should be given to placing the material below the soil Soil 100:1-9. surface. 2. Hamilton, D. F., M. E. C. Graca, and S. D. Verkade. 1981. Critical effects of fertility on root and shoot growth of selected landscape Hibiscus leaf color was found to be sensitive to nitrogen plants. J. of Arboricul. 7:281-290. -

Nephrolepis Cordifolia Global Invasive Species Database (GISD)
FULL ACCOUNT FOR: Nephrolepis cordifolia Nephrolepis cordifolia System: Terrestrial Kingdom Phylum Class Order Family Plantae Pteridophyta Filicopsida Polypodiales Dryopteridaceae Common name herring bone fern (English), tube ladder fern (English), erect sword fern (English), southern sword fern (English), tuber sword fern (English), fish-bone fern (English), lemon butter fern (English), ladder fern (English), narrow swordfern (English), sword fern (English), boston fern (English) Synonym Aspidium cordifolium , (L.) Sw. undated Aspidium pendulum , (Raddi, undated) Aspidium tuberosum , Bory ex Willd. Undated Aspidium volubile , (Sm.) F.M.Bailey, undated Nephrodium pendulum , (Raddi) Desv., undated Nephrodium tuberosum , (Bory ex Willd.) Desv, undated Nephrolepis pendula , (Raddi) J.Sm., undated Nephrolepis radicans , (Burm.f.) Kuhn, undated Nephrolepis tuberosa , (Bory ex Willd.) C.Presl, undated Polypodium cordifolium , L. Similar species Nephrolepis exaltata Summary Nephrolepis cordifolia, commonly known as tuberous sword fern, is a wood fern that typically grows in woodland areas. Dispersal occurs via spores that can be dispersed through wind and water, and through the movement of stolons, tubers and rhizomes. N. cordifolia can form dense stands through its aggressive spread and displace native vegetation. view this species on IUCN Red List Species Description The terrestrial tuberous sword fern is an epiphytic and epilithic plant. Rhizomes are suberect and the fronds upto a meter long. Distinguishing features include the production of tubers. Please follow this link for a description of the species and this link that provides description and key to distinguish alien and native Nephrolepis in the USA Uses Nephrolepis cordifolia is commonly used as an ornamental plant. Global Invasive Species Database (GISD) 2021. Species profile Nephrolepis cordifolia.