Endoscopic Resection Is a Suitable Initial Treatment Strategy for Oxyntic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Company Profile&Company Factsheet
Our customer's success is our pride Our customer's success is our pride JFE Systems, Inc. is a systems integrator established in 1983 as a spin-off from the Information Systems Department of Kawasaki Steel (now JFE Steel Corporation). Over the past 35 years, we have been providing comprehensive services – including planning, design, development, maintenance and operations of corporate information systems – to support our customers in achieving business innovations. In that timeframe, JFE Systems has listed on the Second Section of the Tokyo Stock Exchange and acquired the business of development and maintenance services for JFE Steel and JFE Steel Group companies from EXA Corporation. We currently have 1,800 employees in the whole corporate group and generate sales exceeding 46.5 billion JPY. A Customer-Oriented Spirit With our motto “Customers’ Success is Our Pride,” we strive to achieve the following: ● Provide the best possible solutions through intimate communications with customers to grasp the true nature of the challenges they face ● Present proposals based on a corporate management perspective and provide timely support to customers in their business innovations ● As well as building systems, support customers in fully exploiting such systems to achieve their goals This customer-oriented spirit is the starting point of all our business activities. Experience as an “In-House” Enterprise Systems Integrator Our growth is due to our rich experience in systems development and operations services for JFE Steel, which involved tackling vari- ous technology innovations and quality improvements to maintain mission-critical operations 24 hours a day, 365 days a year. Of these, the Steelworks System Renovation Project, a project currently underway to integrate the backbone systems of all JFE Steel production facilities in Japan, including four steelworks, is the first large-scale project of its kind in the domestic manufacturing industry with the of goal applying the Internet of things (IoT) and other digital technologies to cut lead times and improve quality. -

By Municipality) (As of March 31, 2020)
The fiber optic broadband service coverage rate in Japan as of March 2020 (by municipality) (As of March 31, 2020) Municipal Coverage rate of fiber optic Prefecture Municipality broadband service code for households (%) 11011 Hokkaido Chuo Ward, Sapporo City 100.00 11029 Hokkaido Kita Ward, Sapporo City 100.00 11037 Hokkaido Higashi Ward, Sapporo City 100.00 11045 Hokkaido Shiraishi Ward, Sapporo City 100.00 11053 Hokkaido Toyohira Ward, Sapporo City 100.00 11061 Hokkaido Minami Ward, Sapporo City 99.94 11070 Hokkaido Nishi Ward, Sapporo City 100.00 11088 Hokkaido Atsubetsu Ward, Sapporo City 100.00 11096 Hokkaido Teine Ward, Sapporo City 100.00 11100 Hokkaido Kiyota Ward, Sapporo City 100.00 12025 Hokkaido Hakodate City 99.62 12033 Hokkaido Otaru City 100.00 12041 Hokkaido Asahikawa City 99.96 12050 Hokkaido Muroran City 100.00 12068 Hokkaido Kushiro City 99.31 12076 Hokkaido Obihiro City 99.47 12084 Hokkaido Kitami City 98.84 12092 Hokkaido Yubari City 90.24 12106 Hokkaido Iwamizawa City 93.24 12114 Hokkaido Abashiri City 97.29 12122 Hokkaido Rumoi City 97.57 12131 Hokkaido Tomakomai City 100.00 12149 Hokkaido Wakkanai City 99.99 12157 Hokkaido Bibai City 97.86 12165 Hokkaido Ashibetsu City 91.41 12173 Hokkaido Ebetsu City 100.00 12181 Hokkaido Akabira City 97.97 12190 Hokkaido Monbetsu City 94.60 12203 Hokkaido Shibetsu City 90.22 12211 Hokkaido Nayoro City 95.76 12220 Hokkaido Mikasa City 97.08 12238 Hokkaido Nemuro City 100.00 12246 Hokkaido Chitose City 99.32 12254 Hokkaido Takikawa City 100.00 12262 Hokkaido Sunagawa City 99.13 -

Shikoku Revealed
SShhiikkookkuu RReevveeaalleedd Stone Lanterns at Konpira-San Shrine, Kotohira Your Japan trip at a glance Experience the rich culture, history and nature on offer in Shikoku and surrounding areas with this itinerary, designed for those who want to delve deeper into this fascinating country. Start off in Kobe, a glittering port town that stretches out between the sea and the mountains, before heading to the delightful town of Kurashiki, rich in tradition and craft. Your first destination on Shikoku is Matsuyama, home to one of Japan’s oldest and most famous hot springs. Next on your trip is Kotohira, where you will explore the beautiful Konpira-san shrine. Move on to the city of Takamatsu, and encounter feudal Japan in the castle and traditional gardens. Venture deep into the heart of the Iya Valley, which offers great hiking routes with dramatic natural scenery. Tokushima is your next destination, a city which marks the beginning of the 88 temple pilgrimage around Shikoku and is also home to the most famous dance festival in Japan. At the end of your trip, enjoy the last night of your adventure in the buzzing modern city of Osaka. Kobe The beautiful port city of Kobe may be best known abroad as the source of Kobe beef, but has much more to offer. Its history as one of Japan’s busiest ports has given the city an international feel, which can be appreciated within the lively Chinatown and the district of Western-style residences. The dramatic Rokko Mountain Range rises sharply behind the city, offering hiking trails with spectacular views of the city and the sea. -

Kurashiki Central Hospital
Case Study Remote Backup of Medical Data for Disaster Recovery with HYDRAstor Kurashiki Central Hospital Overview Kurashiki Central Hospital is a hospital with over 1,000 beds in Okayama prefecture, Japan. The hospital had been backing up data onto Linear Tape-Open (LTO) tapes. Master tapes were stored in the hospital building and replicas were stored in an associate hospital located in the same city. However, tape-based backup management and operation was laborious and moving the replica tapes to the associate hospital risked them being stolen or lost in transit. In addition, the backup frequency was only once per week, introducing the risk of losing the latest data when disaster strikes. To resolve Customer these issues, Kurashiki Central Hospital transitioned from tape-based • Kurashiki Central Hospital backup to disk-based backup using NEC HYDRAstor disk-based inline deduplication storage. The hospital was able to achieve fast Industry • Medical – Hospital in Okayama, Japan backup and replication to a robust data center over WAN, maximizing the efficiency of backup management and operation as well as Challenges ensuring business continuity through disaster recovery. • Storage capacity consumption – backup data growth • Backup management – inefficient tape management • Data security – risk of lost or stolen tapes during transport Challenges • Backup frequency – limited to one backup per week Kurashiki Central Hospital had been storing important medical data Solution including health records and accounting data onto LTO tapes for • NEC’s HYDRAstor backup solution backup. The hospital was only able to complete one full backup per ・Fast disk-based backup with inline data deduplication week, from which a master and a replica tape copy was created. -

Educational Institution Kake Educational Institution | 2 | Experience Japanese History and Culture the Cities of OKAYAMA & KURASHIKI
● Okayama University of Science 1-1 Ridai-cho, Kita-ku, Okayama City, Okayama, JAPAN URL: https://www.ous.ac.jp/ KAKE ● Kurashiki University of Science and the Arts 2640 Nishiura, Tsurajima-cho, Kurashiki-city, Okayama, JAPAN EDUCATIONAL URL: https://www.kusa.ac.jp/ INSTITUTION ● Chiba Institute of Science 3 Shiomi-cho, Choshi-city, Ciba, JAPAN URL: http://www.cis.ac.jp/ ● Tamano Institute of Health and Human Services 1-1-20 Chikkou, Tamano-city, Okayama, JAPAN URL: http://www.tamasen.ac.jp/ ● Okayama University of Science Specialized Training College 8-3 Handa-cho, Kita-ku, Okayama-city, Okayama, JAPAN URL: https://www.risen.ac.jp/ ● Okayama University of Science High School 1-1 Ridai-cho, Kita-ku, Okayama City, Okayama, JAPAN ● ● ● URL: http://www.ridaifu.net/ Chiba Institute of Science ● Okayama University of Science Junior High School 1-1 Ridai-cho, Kita-ku, Okayama City, Okayama, JAPAN Okayama University of Science URL: http://www.kake.ac.jp/~info-j/ Kurashiki University of Science and the Arts ● Mikage International Kindergarten 2-15-27 Mikage, Higashinada-ku, Kobe-city, Hyogo, JAPAN URL: http://www.kakemik.jp/ (2020) Founding Philosophy Locations ● Okayama University of Science ● Kurashiki University of Science and the Arts ● Chiba Institute of Science ● Tamano Institute of Health and Human Services ● Okayama University of Science Specialized Training College ● Okayama University of Science High School ● Okayama University of Science Junior High School ● Mikage International Kindergarten 岡山理科大学 岡山理科大学 附属中学校 附属高等学校 Okayama University of Science Jr. High School Okayama University of Science High School Okayama University of Science Chiba Institute of Science To draw out each and every youth’s abilities to their maximum potential, and to educate individuals to 岡山理科大学専門学校 contribute to society as skilled professionals and as Kurashiki University of Science and the Arts Okayama University of Science Specialized Training College members of their communities. -

Organization and History ■
■ Organization and History ■ Organization… …………………………………………… 74 Office Locations…………………………………………… 75 History of JFC……………………………………………… 80 JFC2020 73 Organization Organization Chart of Japan Finance Corporation Board of Directors Evaluation Committee Audit & Supervisory Board Advisory Council to the Governor & CEO Governor & CEO Personnel Committee Audit & Supervisory Board Members’ Office Corporate Governance Committee Deputy Governor ●Secretariat Planning and Micro Business and Agriculture, Forestry, Fisheries Small and Medium Oce Administration Unit Individual Unit and Food Business Unit Enterprise (SME) Unit ●Promotion Oce for Diversity Business Promotion Sub-Unit Business Promotion Sub-Unit Business Promotion Sub-Unit ●Audit and Inclusion ●Business Promotion Department ●Business Promotion ●Business Promotion Department ●Personnel Department ●Loan Planning Department Department Department ●Corporate Governance Oce ●Business Start-Up Support ●Loan Planning Department ●International Operations ●Public Relations Department Department ●Information Planning Department ●System Audit ● (Branch) Micro Business and Department (Representative Oces) Oce Individual Unit, etc. ●(Branch) Small and Medium General Aairs and Planning Credit Sub-Unit Enterprise (SME) Unit, etc. Sub-Unit Environmental Health ●Credit Analysis Department ●Crisis Response ●General Aairs and Coordination Business Loan Sub-Unit ●Revitalization Support Credit Analysis Sub-Unit Finance Department Department ●Environmental Health Department ●Credit Analysis Management ●Corporate -

Changes in Rotavirus Genotypes Before and After Vaccine Introduction
Jpn. J. Infect. Dis., 70, 448–452, 2017 Original Article Changes in Rotavirus Genotypes before and after Vaccine Introduction: a Multicenter, Prospective Observational Study in Three Areas of Japan Takaaki Tanaka1*, Hajime Kamiya2, Kazutoyo Asada3, Shigeru Suga3, Masaru Ido4, Masakazu Umemoto5, Kazunobu Ouchi1, Hiroaki Ito6, Haruo Kuroki7, Takashi Nakano1, and Koki Taniguchi8 1Department of Pediatrics, Kawasaki Medical School, Okayama 700-8505; 2Infectious Disease Surveillance Center, National Institute of Infectious Diseases, Tokyo 162-8640; 3Department of Pediatrics, National Mie Hospital, Mie 514-0125; 4Department of Pediatrics, Mie Chuo Medical Center, Mie 514-1101; 5Umemoto Children’s Clinic, Mie 514-0004; 6Department of Pediatrics, Kameda Medical Center, Chiba 296-8602; 7Sotobo Children’s Clinic, Chiba 299-4503; and 8Department of Virology and Parasitology, Fujita Health University School of Medicine, Aichi 470-1192, Japan SUMMARY: In Japan, monovalent and pentavalent rotavirus (RV) vaccines were approved in 2011 and 2012, respectively. To monitor changes in the RV genotypes before and after vaccine introduction, we performed a prospective observational study among children (< 5 years) with gastroenteritis who test- ed RV-positive on antigen rapid tests. Stool samples were collected from 3 different sites in Japan: Tsu City, Mie Prefecture; Kurashiki City, Okayama Prefecture; and Isumi City, Chiba Prefecture. RV geno- types were determined using reverse transcription-polymerase chain reaction. In Tsu City, G3P[8] was dominant (61.0–77.1%) before vaccine introduction, but decreased after introduction. Meanwhile, in an inverse proportion to the decrease in G3P[8], G1P[8] increased until the 2013/14 season, when a sud- den predominance of G2P[4] (100%) occurred. -

Local Communities
Contributing to Societal Development Local Communities JFE Standards of Business Conduct (Excerpt) Education at Elementary Schools JFE Steel conducts plant tours for students at nearby (3) Work with communities elementary schools. In addition, company employees Actively contribute to host communities as a good corporate visit schools to give lectures on iron and steelmaking Management citizen by emphasizing harmony and cooperation. processes, the features of steelworks, environmental initiatives and other topics to deepen understanding of the steel industry. These lectures have been given to over 165 classes since its start in FY2012. In FY2017, Local Activities the company conducted the first class at a school for Host Communities hearing impaired children. Every year, the JFE Group opens its manufacturing Protecting the Global Environment facilities to residents in local host communities for demonstrations, tours and other events. ■ On-site Events in FY2018 Location Event Date Attendees East Japan Keihin Community May 26 46,000 Works, Keihin Festival East Japan JFE Chiba Festival October 28 40,000 Works, Chiba West Japan JFE West Japan Festival May 13 59,000 Contributing to Societal Development Works, Fukuyama in Fukuyama Visiting lecturer at Samugawa Elementary School in Chiba City West Japan JFE West Japan Festival November 3 110,000 Works, Kurashiki in Kurashiki Handa Community Chita Works November 10 21,000 Industrial Festival Support for External Organizations Contributing to the realization of a sustainable society is a key management concern for the JFE Group, which actively seeks to address issues in collaboration with external groups and NGOs in pursuing solutions for the 17 SDGs. Keihin Community Festival UN World Food Programme The JFE Group seeks to resolve the global hunger issue In addition, on-site recreational facilities are made by supporting the cause and activities of the Japan available for community sports such as soccer, Association for the World Food Programme*. -
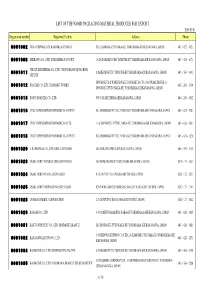
LIST of the WOOD PACKAGING MATERIAL PRODUCER for EXPORT 2005/03/01 Registration Number Registered Facility Address Phone
LIST OF THE WOOD PACKAGING MATERIAL PRODUCER FOR EXPORT 2005/03/01 Registration number Registered Facility Address Phone 0001002 ITOS CORPORATION KAMOME-JIGYOSHO 62-1 KAMOME-CHO NAKA-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐622‐1421 0001008 ISHIKAWA CO., LTD. YOKOHAMA FACTORY 18-24 DAIKOKU-CHO TSURUMI-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐521‐6171 THE IZUMI EXPRESS CO., LTD. TOKYO BRANCH, PACKING 0001011 8 DAIKOKU-FUTO TSURUMI-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐504‐9431 CENTER HONMOKU B-2 WARE HOUSE, HONMOKU D-CFS 1 GO WARE HOUSE 3-1 0001012 INAGAKI CO., LTD. HONMOKU WORKS 045‐260‐1160 HONMOKU-FUTO NAKA-KU YOKOHAMA-SHI KANAGAWA, JAPAN 0001013 INOUE MOKUZAI CO., LTD. 895-3 SYAKE EBINA-SHI KANAGAWA, JAPAN 046‐236‐6512 0001016 UTOC CORPORATION HONMOKU B-1 OFFICE B-1, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐621‐5781 0001017 UTOC CORPORATION HONMOKU D-5 CFS 1-16, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐623‐1241 0001018 UTOC CORPORATION HONMOKU B-3 OFFICE B-3, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐621‐6226 0001020 A.B. SHOHKAI CO., LTD. EBINA-JIGYOSHO 642 NAKANO EBINA-SHI KANAGAWA, JAPAN 046‐239‐0133 0001023 OSAKI CORP. TATEBAYASHI EIGYOUSHO 358 NOBE-MACHI TATEBAYASHI-SHI GUNMA, JAPAN 0276‐74‐6531 0001024 OSAKI CORP. OYAMA EIGYOUSHO 4-18-39 JYOUTOU OYAMA-SHI TOCHIGI, JAPAN 0285‐22‐1211 0001025 OSAKI CORP. NISHINASUNO EIGYOUSHO 429-9 NIKU-MACHI NISHINASUNO-CHO NASU-GUN TOCHIGI, JAPAN 0287‐37‐7161 0001028 OYAMA LUMBER CORPORATION 2-7-26 TENJIN-CHO OYAMA-SHI TOCHIGI, JAPAN 0285‐22‐0022 0001029 KAGAMI CO., LTD. -

Infliximab Versus Intravenous Immunoglobulin for Refractory
www.nature.com/scientificreports OPEN Infiximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, Received: 30 June 2017 Accepted: 5 December 2017 randomized, open-label, active- Published: xx xx xxxx controlled, parallel-group, multicenter trial Masaaki Mori1,15, Takuma Hara1,16, Masako Kikuchi2, Hiroyuki Shimizu3, Tomoyuki Miyamoto4, Satoru Iwashima 5,17, Tatsuya Oonishi6, Kunio Hashimoto7, Norimoto Kobayashi8, Kenji Waki9, Yasuo Suzuki10, Yoshikazu Otsubo11, Hiroshi Yamada12, Chikao Ishikawa12, Taichi Kato13 & Shigeto Fuse14 We compared the efcacy and safety of infiximab with intravenous immunoglobulin (IVIG), a standard therapy, in a phase 3 trial (NCT01596335) for Japanese patients with Kawasaki disease (KD) showing persistent fever after initial IVIG. Patients with initial IVIG-refractory KD, aged 1–10 years, received a single dose of IV infiximab 5 mg/kg or IV polyethylene glycol-treated human immunoglobulin (VGIH) 2 g/kg on day 0. Primary outcome was defervescence rate within 48 h after the start of treatment. Safety was evaluated through day 56. Overall, 31 patients were randomized (infiximab, n = 16; VGIH, n = 15); 31.3% and 60.0% patients discontinued due to worsening KD. Defervescence rate within 48 h was greater with infiximab (76.7%) than VGIH (37.0%) (p = 0.023), and defervescence was achieved earlier with infiximab (p = 0.0072). Coronary artery lesions occurred in 1 (6.3%) and 3 (20.0%) patients receiving infiximab and VGIH, respectively, up to day 21. Adverse events occurred in 15 (93.8%) and 15 (100.0%) patients in the infiximab and VGIH groups, respectively. No serious adverse events in the infiximab group and one in the VGIH group were observed. -

OKAYAMA, KURASHIKI and SETO-OHASHI BRIDGE PAGE 1/ 5
OKAYAMA, KURASHIKI and SETO-OHASHI BRIDGE PAGE 1/ 5 PG-602 OKAYAMA, KURASHIKI and SETO-OHASHI BRIDGE Okayama (岡山) is one of the major commercial, industrial and Kurashiki (倉敷) is an old merchants town near Okayama. In cultural cities in the Chugoku District in western Japan. It is feudal days, it thrived as a port for the shipment of rice; several nationally known for its celebrated Korakuen Garden. It also old rice granaries remain there. Its olden time atmosphere and a serves as a main gateway to Inland Sea National Park and variety of museums lure many visitors to Kurashiki. Shikoku Island. Okayama Airport Bizen- Shin- Soja Ichinomiya bus Kurashiki JR Kibi Line Aioi Kibitsu Okayama JR Shinkansen Line To Hiroshima JR Sanyo Line To Himeji, Kyoto Kurashiki Higashi- bus Okayama Imbe Ako Line Kojima Washuzan Hill Uno bus To Shikoku Is. Access: By Air from Tokyo (Haneda Airport) To Operated by Time required Daily Flights One-way fare Access airport – downtown 35 min. to Okayama Sta. by bus (¥680) ANA 45 min. to Kurashiki Sta. by Airport Okayama Toll free: 0120-029- 1 hr. 20 min. 5 ¥25,500 - ¥27,500 Limousine bus (¥1,000) 222 *Only 4 bus services per day. Please confirm the time table. By Train *Number of flights and fare may change by season. To From Type of Transportation Time required Daily runs One-way fare Okayama Tokyo JR Shinkansen (By Hikari) 3 hrs. 53 min. - 4 hrs. 10 min. 32 ¥16,360 JR Shinkansen (By Nozomi) 3 hrs. 12 min. - 3 hrs. 18 min. -

Mizushima Training Center Training Center Information
Mizushima Training Center Training Center Information Contact Information QUICK LINKS Mizushima Solution Center 36 -8 Tsurajima Tsurajima-Cho ▪ Mizushima Office Location Map Kurashiki -Shi Okayama-Ken ▪ Mizushima Recommended Hotels Japan ▪ Global Training Center Directory ▪ Locate an Emerson Office Phone: +81-86-445-7270 ▪ Online Course Catalog (MyCONNECT) Educational Services Mizushima Resources ▪ Asia Pacific Inquiry Form ▪ Asia Pacific Training Offerings Catalog ▪ Emerson Japan Website Educational Services [email protected] www.Emerson.com/Education Emerson Educational Services is accredited by the International Association for Continuing Education and Training (IACET) and is accredited to issue the IACET CEU Mizushima Office Location Map Directions to the Emerson Facility Educational Services [email protected] www.Emerson.com/Education Emerson Educational Services is accredited by the International Association for Continuing Education and Training (IACET) and is accredited to issue the IACET CEU Mizushima Recommended Hotels Please contact the hotel for updated rates. Domi In Kurashiki Hotel Photo Gallery Access: 7 minutes from JR Kurashiki station South East 3-21-11 Achi Kurashiki-Shi Okayama Phone: +81-86-426-5489 Hotel Website Kurashiki Royal Art Hotel Hotel Amenities 3-21-19 Achi Kurashiki City Okayama 710-0055 Japan Kurashiki Royal Art Hotel offers every guest extraordinary Phone: +81-86-423-2400 comfort, located near the heart of Kurashiki, just three Fax: +81-86-423-2401 minutes' walk from Bikan district, which maintains the old Hotel Website charm of Edo Period. As you step into naturally sun-lit entrance hall, you cannot fail to feel brightened. You can feel this is just the beginning of your wonderful stay in Kurashiki.