The Influence of Water Motion on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cloughey Village Plan
CLOUGHEY Integrated Village Plan 2018-2023 CLOUGHEY Integrated Village Plan 2018-2023 1 CONTENTS 1 Introduction to Cloughey Village Plan Page 5 2 The Village of Cloughey Page 6 3 Cloughey Village Plan – Consultation Process Page 12 4 Analysis and Findings Page 15 5 Vision and Overarching Aims Page 18 6 Detail of Village Projects Page 20 7 Delivery of Cloughey Village Action Plan Page 28 8 Appendices Page 34 Appendix A – Community Survey Results Appendix B – Tracking and Monitoring Template 2 CLOUGHEY Integrated Village Plan 2018-2023 CLOUGHEY Integrated Village Plan 2018-2023 3 1 Introduction to Cloughey Village Plan 1.1 BACKGROUND TO VILLAGE PLANNING A range of actions has been delivered which have, for example, improved access to the beach As part of the Rural Development Programme 2014–2020, through the installation of boardwalks. The tennis Ards and North Down Borough Council provided support courts have been enhanced to provide three all-weather to help villages within the Council area revise and update state-of-the-art courts. There is improved sharing their village plans. These plans identify the specific needs of information through the Cloughey website and social of each village and set out a range of agreed actions media pages and organised activities and events such to be delivered over the next five years that will help as the beach clean-up. improve the village for everyone. The local community has faced challenges in progressing The village plan is a working document that has been actions which are under the responsibility of statutory developed through a process of engagement with agencies or require the support of statutory agencies. -

Official Report
Friday Volume 34 7 November 2008 No WA 3 OFFICIAL REPORT (HANSARD) CONTENTS Written Answers to Questions Office of the First Minister and deputy First Minister [p189] Agriculture and Rural Development [p203] Culture, Arts and Leisure [p212] Education [p221] Employment and Learning [p243] Enterprise, Trade and Investment [p249] Environment [p253] Finance and Personnel [p264] Health, Social Services and Public Safety [p276] Regional Development [p303] Social Development [p316] Assembly Commission [p336] Written Answers [p337] £5.00 This publication contains the written answers to questions tabled by Members. The content of the responses is as received at the time from the relevant Minister or representative of the Assembly Commission and has not been subject to the official reporting process or changed in any way. This document is available in a range of alternative formats. For more information please contact the Northern Ireland Assembly, Printed Paper Office, Parliament Buildings, Stormont, Belfast, BT4 3XX Tel: 028 9052 1078 ASSEMBLY MeMBerS Adams, Gerry (West Belfast) McCarthy, Kieran (Strangford) Anderson, Ms Martina (Foyle) McCartney, Raymond (Foyle) Armstrong, Billy (Mid Ulster) McCausland, Nelson (North Belfast) Attwood, Alex (West Belfast) McClarty, David (East Londonderry) Beggs, Roy (East Antrim) McCrea, Basil (Lagan Valley) Boylan, Cathal (Newry and Armagh) McCrea, Ian (Mid Ulster) Bradley, Dominic (Newry and Armagh) McCrea, Dr William (South Antrim) Bradley, Mrs Mary (Foyle) McDonnell, Dr Alasdair (South Belfast) Bradley, P -

Sanitary Survey Review for Strangford Lough
Sanitary Survey Review for Strangford Lough Produced by AQUAFACT International Services Ltd On behalf of The Food Standards Agency in Northern Ireland March 2021 Aquafact International Services Ltd. 12 Kilkerrin park Tuam Road Galway city www.aquafact.ie [email protected] Table of Contents Glossary ......................................................................................................... 1 1. Executive Summary................................................................................. 5 2. Overview of the Fishery/Production Area ............................................. 7 2.1. Location/Extent of Growing/Harvesting Area .......................................... 7 2.2. Description of the Area ......................................................................... 11 3. Hydrography/Hydrodynamics .............................................................. 15 3.1. Simple/Complex Models ....................................................................... 15 3.2. Depth .................................................................................................... 16 3.3. Tides & Currents ................................................................................... 18 3.4. Wind and Waves................................................................................... 30 3.5. River Discharges .................................................................................. 35 3.6. Rainfall Data ......................................................................................... 39 3.6.1. Amount -

The Belfast Gazette, November 23, 1923. 517
THE BELFAST GAZETTE, NOVEMBER 23, 1923. 517 Ballintlieve, Ballintogher, Ballyalton, Bally- lessan, Ballyniullan, Ballynahatty, Bally- beg, Ballybrannagh Lower, Ballybrannagh skeagh, Drumbeg, Drumbo (that portion north Upper, Ballyclander Lower, Ballyclander of the road from Shaw's Bridge to Lisburn), Upper, Ballycruttle, Ballyculter Lower, Bally- Edenderry, Largymore, Lisnatrunk (Parish of culter Upper, Ballyedock Lower, Ballyedock Blaris), Lisnatrunk (Parish of Lambeg), and Upper, Ballyhornan, Ballyhosset, Ballyhosset Tullynacross, in the barony of Castlereagh Miltown, Ballylenagh, Ballymeiiagh, Bally- Upper, in the Administrative County of Down; murry, Ballynagarrick, Ballynagross Lower, the townlands of Lambeg South, Lisnagarvey, Ballynagross Upper, Ballyorgan, Ballyrenaii, Old Warren, and Tonagh, in the barony of Ballysallagh, Ballystokes, Ballysugagh, Bally- Massereene Upper, in the Administrative trustan, Ballywalter, Ballywoodan, Bishops- County of Antrim, and the Urban District of court, Cargagh, Carrintaggart, Carrowbaghran, Eisburn, in the baronies of Massereene Upper Carrowcarlin, Carrownacaw, Carrowvanny, and Castlereagh Upper in the Administrative Castle Island, Castlemahon, Castleward, Church County of Antrim, the townlands of Bally- Bailee, Cloghy, Coney Island, Corbally, Dillin, finaghy, Derryaghy, Dunmurry, Killeaton, Dunsfort. Ferryquarter, Glebe, Isle M'Cricket, Kilmakee, Lambeg North, Malone Upper and Jordan's Crew, Kilclief, Kildare's Crew, Kil- Old Forge, in the barony of Belfast Upper in lard Upper, Killard Lower, -

The Down Rare Plant Register of Scarce & Threatened Vascular Plants
Vascular Plant Register County Down County Down Scarce, Rare & Extinct Vascular Plant Register and Checklist of Species Graham Day & Paul Hackney Record editor: Graham Day Authors of species accounts: Graham Day and Paul Hackney General editor: Julia Nunn 2008 These records have been selected from the database held by the Centre for Environmental Data and Recording at the Ulster Museum. The database comprises all known county Down records. The records that form the basis for this work were made by botanists, most of whom were amateur and some of whom were professional, employed by government departments or undertaking environmental impact assessments. This publication is intended to be of assistance to conservation and planning organisations and authorities, district and local councils and interested members of the public. Cover design by Fiona Maitland Cover photographs: Mourne Mountains from Murlough National Nature Reserve © Julia Nunn Hyoscyamus niger © Graham Day Spiranthes romanzoffiana © Graham Day Gentianella campestris © Graham Day MAGNI Publication no. 016 © National Museums & Galleries of Northern Ireland 1 Vascular Plant Register County Down 2 Vascular Plant Register County Down CONTENTS Preface 5 Introduction 7 Conservation legislation categories 7 The species accounts 10 Key to abbreviations used in the text and the records 11 Contact details 12 Acknowledgements 12 Species accounts for scarce, rare and extinct vascular plants 13 Casual species 161 Checklist of taxa from county Down 166 Publications relevant to the flora of county Down 180 Index 182 3 Vascular Plant Register County Down 4 Vascular Plant Register County Down PREFACE County Down is distinguished among Irish counties by its relatively diverse and interesting flora, as a consequence of its range of habitats and long coastline. -

An Assessment of Aquatic Radiation Pathways in Northern Ireland
An Assessment of Aquatic Radiation Pathways in Northern Ireland Research Commissioned by the Scotland and Northern Ireland Forum For Environmental Research (SNIFFER) and Environment and Heritage Service (EHS). SNIFFER Contract AIR(99)03 CEFAS Contract C1187 Environment Report 17/01 1 Environment Report RL 20/02 An Assessment of Aquatic Radiation Pathways in Northern Ireland The Centre for Environment, Fisheries and Aquaculture Science Lowestoft Laboratory Pakefield Road Lowestoft Suffolk NR33 0HT D. L. Smith, B. D. Smith, A. E. Joyce and I. T. McMeekan December 2002 The work described in this report was carried out on behalf of the Scotland and Northern Ireland Forum For Environmental Research (SNIFFER) and Environment and Heritage Service (EHS), as part of CEFAS contract number C1187 (SNIFFER contract number AIR(99)03) . 2 CONTENTS Page EXECUTIVE SUMMARY 6 1. INTRODUCTION 8 2. THE HABITS SURVEY 9 2.1 Survey aims 9 2.2 Survey area 9 2.3 Conduct of the survey 9 3. LOCAL FISHING EFFORT 11 3.1 The fishing industry 11 3.2 Fishing areas 11 4. INTERNAL RADIATION EXPOSURE PATHWAYS 12 4.1 Fish 12 4.1.1 Inshore and offshore fishing 12 4.1.2 Angling 12 4.2 Crustaceans 13 4.2.1 Nephrops 13 4.2.2 Crabs and lobsters 13 4.3 Molluscs 14 4.3.1 Periwinkles and whelks 14 4.3.2 Oysters and mussels 14 4.3.3 Scallops and clams 14 4.3.4 Razor fish and squid 15 5. EXTERNAL RADIATION EXPOSURE PATHWAYS 15 5.1 Beach and coastal area activities 15 5.2 Watersport activities 16 5.3 Handling 16 6. -

Foster's Tern Sterna Forsteri
Foster’s Tern Sterna forsteri (0, 2, 4). (Breeds North America, winters south to Caribbean and northern Central America). 1983 One: Bann Estuary, Coleraine, County Londonderry, 3rd - 5th December (Davy Hunter et al.). NIBRC 1987. 1984 One: Groomsport, County Down, 19th - 20th March (Anthony McGeehan et al.). NIBRC 1987. One: Quoile Pondage NNR, County Down, 13th April - 1st June (R.J. Bleakley et al.). NIBRC 1987. 1990 One: Adult. Brigg's Rocks, Groomsport, County Down, 11th February. NIBA 1992. Not published in Irish Bird Report. 1991 One: Cloghy Rocks, County Down, 6th January. NIBA 1993. One: Adult. Outer Ards peninsula between Millisle and Ballywalter, County Down, 12th January to 8th March (Anthony McGeehan et al.). Photos IrishBirding News 1: 3, p.88 and British Birds 84: 326. 1992 One: Drumfad Bay, Ballywalter Road, Millisle, County Down, 6th January - 8th March (Anthony McGeehan et al.). NIBA 1993. One: Adult. Ballyferris / Millisle / Ballywalter, County Down, 8th November into 1993 (Anthony McGeehan et al.). NIBA 1993. 1993 One: Adult. Ballyferris / Millisle / Ballywalter, County Down, from 1992 remained to 23rd February (Anthony McGeehan et al.). NIBA 1994. 2003 One: Bann Estuary, Coleraine, County Londonderry, 22nd April - 4th May (Jeff Larkin, Laurence Morgan et al.). NIBA 2006. One: Dundrum Inner Bay, Newcastle, County Down, 7th May (A. Cowie, N. Willis). Irish Birds 8: 373-394. Milne, P. & McAdams, D.G. 2008a. Irish Rare Bird Report 2005, 2004 One: Adult. Watched for 15 minutes, Killyleagh Harbour, Killyleagh, Strangford Lough, County Down, 7th February (Joe Lamont, Ivan Quail), same Tully Hill Bay, Strangford, County Down, 8th February (Walter Veale et al.), same Cloghy Rocks Nature Reserve, Strangford, County Down, 8th February (Richard Weyl, Wilton Farrelly) same Kilclief Bay, Kilclief Village, Strangford, County Down, 17th March (Richard Weyl), same Briggs Rocks, Groomsport, County Down, 27th - 28th March (Richard Weyl et al.) and finally at Cockle Island, Groomsport, County Down on 28th March (Clive Mellon, Dave Allen et al.). -
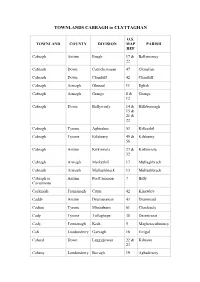
Townlands Cabragh to Clyttaghan Adobe
TOWNLANDS CABRAGH to CLYTTAGHAN O.S. TOWNLAND COUNTY DIVISION MAP PARISH REF Cabragh Antrim Enagh 17 & Ballymoney 22 Cabragh Down Carrickcrossan 47 Clonallan Cabragh Down Clonduff 42 Clonduff Cabragh Armagh Glenaul 11 Eglish Cabragh Armagh Grange 8 & Grange 12 Cabragh Down Ballyworfy 14 & Hillsborough 15 & 21 & 22 Cabragh Tyrone Aghnahoe 53 Killeeshil Cabragh Tyrone Kilskeery 49 & Kilskeery 56 Cabragh Antrim Kirkinriola 27 & Kirkinriola 32 Cabragh Armagh Markethill 17 Mullaghbrack Cabragh Armagh Mullaghbrack 13 Mullaghbrack Cabragh or Antrim PortCammon 7 Billy Cavanmore Cackinish Fermanagh Crum 42 Kinawley Caddy Antrim Drumanaway 43 Drummaul Cadian Tyrone Minterburn 61 Clonfeacle Cady Tyrone Tullaghoge 38 Desertcreat Cady Fermanagh Kesh 5 Magheraculmoney Cah Londonderry Garvagh 18 Errigal Cahard Down Leggygowan 22 & Kilmore 23 Caheny Londonderry Bovagh 19 Aghadowey Caherty Antrim Ballyclug 33 Ballyclug Cahery Londonderry Keady 10 Drumachose Cahoo Tyrone Tullaghoge 38 & Donaghenry 39 Cahore Londonderry Draperstown 40 Ballynascreen Cahore Fermanagh Ederny 6 Drumkeeran Caldanagh Antrim Dunloy 22 & Finvoy 23 Caldragh Fermanagh Kinawley 38 Kinawley Caldrum Tyrone Favour Royal 59 Clogher Caldrum Glebe Fermanagh Rahalton 15 Inishmacsaint Caledon Tyrone Caledon 67 & Aghaloo 71 Calf Island Down Kilmood 17 Ardkeen Calhame Antrim Ballynure 45, Ballynure 46, 51 & 52 Calheme Antrim Stranocum 17 Ballymoney Calheme Tyrone Edymore 5 Camus Calkill Tyrone Castletown 25 & Cappagh 34 Calkill Fermanagh Killesher 26 & Killesher 32 Callagheen Fermanagh Inishmacsaint -

Premises Name House Number Street Town Post Code FBO Type
Premises Name House Number Street Town Post Code FBO Type Contact Number How to order How to pay Door Step Delivery Additional Information Ballymoat Farm Shop 4 Moat Road Ballyhalbert BT22 1DJ Supermarket / other 07817 703 650 Telephone Cash or pre paid into Only for elderly and Open by appointment only. Flexible. Sells food retailer bank account vulnerable Frozen Meats Lamb Beef (blast frozen Pies and Potatoes. Pre Pay. Contact on mobile for bank details and sort code. Would be prepared to deliver to elderly and vulnerable only during crisis in radius of Ballywalter, Portavogie and Ballyhalbert SPAR 24-28 High Street Ballyhalbert BT22 1BL Supermarket / other 028 4275 8768 Telephone before Card & Cash Yes Two delivery options, the owner of the Spar BALLYHALBERT food retailer 9.30am does deliveries and the Ballyhalbert Community Page on Facebook are also collecting and doing deliveries. Pre Packed items only. The current opening hours are about to change from 7am to 10pm BALLYWALTER POST 55 Main Street Ballywalter BT22 2PQ Confectioners / 028 42758212 Telephone or message Preferably card or leave Yes Opening hours 8am to 8pm. No Post office they OFFICE (XL STOP & SHOP) Newsagents the facebook page cash out now do My Hermes Delivery Service. Shop sells essentials like bread milk papers etc. Deliveries daily as and when they can. Prefer that a family member pays for items for the week at the start of the week by card CAFE ARDMORE AND 35 Main Street Ballywalter BT22 2PQ Cafes / Restaurants / 028 42758262 Telephone Preferably card but can Yes on Tuesdays and Cafe Ardmore closed and no takeaways. -

Download (Pdf)
app, for more information at your fingertips. your at information more for app, /nationaltrust.ni find us on Facebook, or download the Visit Strangford Strangford Visit the download or Facebook, on us find knowledge to enhance your experience. Visit our website, website, our Visit experience. your enhance to knowledge www.nationaltrust.org.uk www.nationaltrust.org.uk visit information, more For Centres where our staff will provide you with all the the all with you provide will staff our where Centres Plan your trip by calling in to the local Visitor Information Information Visitor local the to in calling by trip your Plan at a pace that suits you. suits that pace a at Galloway. You can easily put together an inspiring visit to Ards. or to download from the website. the from download to or canoeing, birdwatching, boat trips, cycling, and walks all all walks and cycling, trips, boat birdwatching, canoeing, Explore/ of Man and the Mull of of Mull the and Man of Millisle, Newtownards and Portaferry, in brochure format format brochure in Portaferry, and Newtownards Millisle, trails, arts and crafts, as well as many activities including including activities many as well as crafts, and arts trails, The following suggestions each take around half a day - year-round must-see. year-round Mourne Mountains, Isle Isle Mountains, Mourne Donaghadee, Downpatrick, Greyabbey, Killyleagh, Killyleagh, Greyabbey, Downpatrick, Donaghadee, beaches, amazing wildlife, captivating gardens, coastal coastal gardens, captivating wildlife, amazing beaches, Rowallane -

Hand Amendments/Changes to Vfr Charts Belfast Tma Class D Airspace 6°30'0"W 6°0'0"W 5°30'0"W
HAND AMENDMENTS/CHANGES TO VFR CHARTS BELFAST TMA CLASS D AIRSPACE 6°30'0"W 6°0'0"W 5°30'0"W . R r h e COLERAINE Ballybogy us iv Dervock B 1368 R n du Stranocum n 1267 le G Kirkhills (332) KU1808 BALLYMONEY Cushendall 1667 Trostan Kilraghts Garron Point Ballylintagh 1808 1260 1302 Bendooragh (329) 1049 1482 SCOTTISH FIR (331) Hunters Point Mullaghmore (410) Dunloy 1538 55°0'0"N Finvoy (329) 55°0'0"N Clogh Mills Carnlough EGAC StraidkillyB elfast City LONDON FIR 1474 Glenarm Point Moneydig McLaughlins Clogh Corner 676 1431 130.850 Martinstown 04/22 1017 Maidens KILREA Glarryford Bovedy (328) McGregor's Corner 1250 1829 Fl (3) W 15s River Main 96 15 (76) R Sheddings i v Craigs e Swatragh r Carncastle Tamlaght Buckna B 555 A a M O'Crilly (394) 568 T T n Cullybackey 1260 FAS n EL (394) B 05 1437 LARNE -FL1 Upperlands Clady Portglenone 00FT 653 BALLYMENA Agnews 35 Culnady 05 Hill D AHOGHILL - FL1 000FT 1555 D 2 869 Larne A 1338 KU1555 Gulladuff TM Lough B FAST (328) E BEL 425 D L 1200 2 FA (403) 00 S Bellaghy L (329) 0 T 1158 F T Curran T M Beg D SFC /F A R - F Black Head L1 T L Fl W 3s 0 Castledawson Moneyglass C 1 5 T 05 1224 148 671 D (53) S (335) B A MAGHERAFELT Toome A RANDALSTOWN (663) E T 1017 1 L C 0 F 5 FA 0 L 0 S T 5 0F S 3 E T T - Knockagh /2 C A 0 B CARRICKFERGUS 0 TA F 0 Staffordstown 0 L ANTRIM 0 0 Mew Island Ballyronan EGAA F E 2 T Fl (4) W 30s B The Loup Belfast KU1744 Helen's Bay D Copel1a2n2d Isla(n1d22 ) Aldergrove BANGOR OY NDB HB NDB 128.500 332 KHz 382 420 KHz 07/25 (1327097) 2780 TDME Donaghadee Coagh 354 DONAGHADEE -

Intertidal Fish Traps from Ireland: Some Recent Discoveries in Lough Swilly, Co
J Mari Arch (2015) 10:117–139 DOI 10.1007/s11457-015-9146-z ORIGINAL PAPER Intertidal Fish Traps from Ireland: Some Recent Discoveries in Lough Swilly, Co. Donegal 1 1 1 P. Montgomery • W. Forsythe • C. Breen Published online: 2 September 2015 Ó Springer Science+Business Media New York 2015 Abstract Fish traps are one of the most widespread and enduring features of the maritime landscape. Recent research in Ireland has identified a great number of traps, most of which date from the early to late medieval periods. This paper presents the findings of a recent survey of Lough Swilly in north-western Ireland where a series of fish traps offers new insights into the survival, diversity and role of these sites in the post-medieval period. Keywords Intertidal fish traps Á Ireland Á Maritime resources Á Post-medieval Introduction Fish traps have been used for catching fish in a range of environments (fluvial, estuarine and maritime) from the Mesolithic to the modern era. These fixed traps present a barrier to the migration of fish, which are directed via arms (or leaders) to the apex (or eye) of the trap where they are collected. Those situated in a marine environment exploit the move- ment of fish to the shallows to feed on a rising tide and are most commonly designed to catch them on the ebb tide. They represent one of the earliest complex constructions made by humans that survive in the archaeological record. Their use as a means of catching large numbers of fish has made them central to economies, and has had a major role in the development of trade and enterprise among communities (e.g.