Complex Shoulder Instability: the Role of the Latarjet Coracoid Transfer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Netter's Musculoskeletal Flash Cards, 1E
Netter’s Musculoskeletal Flash Cards Jennifer Hart, PA-C, ATC Mark D. Miller, MD University of Virginia This page intentionally left blank Preface In a world dominated by electronics and gadgetry, learning from fl ash cards remains a reassuringly “tried and true” method of building knowledge. They taught us subtraction and multiplication tables when we were young, and here we use them to navigate the basics of musculoskeletal medicine. Netter illustrations are supplemented with clinical, radiographic, and arthroscopic images to review the most common musculoskeletal diseases. These cards provide the user with a steadfast tool for the very best kind of learning—that which is self directed. “Learning is not attained by chance, it must be sought for with ardor and attended to with diligence.” —Abigail Adams (1744–1818) “It’s that moment of dawning comprehension I live for!” —Calvin (Calvin and Hobbes) Jennifer Hart, PA-C, ATC Mark D. Miller, MD Netter’s Musculoskeletal Flash Cards 1600 John F. Kennedy Blvd. Ste 1800 Philadelphia, PA 19103-2899 NETTER’S MUSCULOSKELETAL FLASH CARDS ISBN: 978-1-4160-4630-1 Copyright © 2008 by Saunders, an imprint of Elsevier Inc. All rights reserved. No part of this book may be produced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording or any information storage and retrieval system, without permission in writing from the publishers. Permissions for Netter Art figures may be sought directly from Elsevier’s Health Science Licensing Department in Philadelphia PA, USA: phone 1-800-523-1649, ext. 3276 or (215) 239-3276; or e-mail [email protected]. -
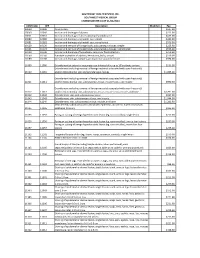
SOUTHWEST HEALTH SYSTEM, INC. SOUTHWEST MEDICAL GROUP CHARGEMASTER AS of 01/01/2021 CDM Code CPT Description Modifiers Fee 10040
SOUTHWEST HEALTH SYSTEM, INC. SOUTHWEST MEDICAL GROUP CHARGEMASTER AS OF 01/01/2021 CDM Code CPT Description Modifiers Fee 10040 10040 Acne surgery $147.00 10060 10060 Incision and drainage of abscess $212.00 10061 10061 Incision and drainage of abscess(multiple\complicated $422.00 10080 10080 Incision and drainage of pilonidal cyst; simple $340.00 10081 10081 Incision and drainage of pilonidal cyst; complicated $576.00 10120 10120 Incision and removal of foreign body, subcutaneous tissues; simple $225.00 10121 10121 Incision and removal of foreign body, subcutaneous tissues; complicated $513.00 10140 10140 Incision and drainage of hematoma, seroma or fluid collection $279.00 10160 10160 Puncture aspiration of abscess, hematoma, bulla, or cyst $179.00 10180 10180 Incision and drainage, complex, postoperative wound infection $594.00 11000 11000 Debridement of extensive eczematous or infected skin; up to 10% of body surface $133.00 Debridement including removal of foreign material associated with open fracture(s) 11010 11010 and/or dislocation(s); skin and subcutaneous tissues $1,085.00 Debridement including removal of foreign material associated with open fracture(s) 11011 11011 and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, and muscle $996.00 Debridement including removal of foreign material associated with open fracture(s) 11012 11012 and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, muscle, and bone $2,791.00 11042 11042 Debridement; skin, and subcutaneous tissue $197.00 11043 11043 Debridement; skin, -

Subacromial Decompression in the Shoulder
Subacromial Decompression Geoffrey S. Van Thiel, Matthew T. Provencher, Shane J. Nho, and Anthony A. Romeo PROCEDURE 2 22 Indications P ITFALLS ■ Impingement symptoms refractory to at least • There are numerous possible 3 months of nonoperative management causes of shoulder pain that can ■ In conjunction with arthroscopic treatment of a mimic impingement symptoms. All potential causes should be rotator cuff tear thoroughly evaluated prior to ■ Relative indication: type II or III acromion with undertaking operative treatment clinical fi ndings of impingement of isolated impingement syndrome. Examination/Imaging Subacromial Decompression PHYSICAL EXAMINATION ■ Assess the patient for Controversies • Complete shoulder examination with range of • Subacromial decompression in motion and strength the treatment of rotator cuff • Tenderness with palpation over anterolateral pathology has been continually acromion and supraspinatus debated. Prospective studies • Classic Neer sign with anterolateral shoulder have suggested that there is no difference in outcomes with and pain on forward elevation above 90° when without subacromial the greater tuberosity impacts the anterior decompression. acromion (and made worse with internal rotation) • Subacromial decompression • Positive Hawkins sign: pain with internal rotation, performed in association with a forward elevation to 90°, and adduction, which superior labrum anterior- causes impingement against the coracoacromial posterior (SLAP) repair can potentially increase ligament postoperative stiffness. ■ The impingement test is positive if the patient experiences pain relief with a subacromial injection of lidocaine. ■ Be certain to evaluate for acromioclavicular (AC) joint pathology, and keep in mind that there are several causes of shoulder pain that can mimic impingement syndrome. P ITFALLS IMAGING • Ensure that an axillary lateral ■ Standard radiographs should be ordered, view is obtained to rule out an os acromiale. -

FSH Chrgmaster 12-2018
Description Q Code CPTCode Rev Code Cost Markup Flatfee Markup% Billable Billable Fee 300-399 MG/ML IODINE CONCENTRATE Q9967 Q9967 320 50.00 100.00 50.00 ABDOMINO-VAGINAL VESICAL NECK SUSPENSION, WITH OR WITHOUT ENDOSCOPIC 51845 51845 360 100.00 0.00 CONTROL (EG, STAMEY, RAZ, MODIFIED PEREYRA) ABLATION, ONE OR MORE LIVER TUMOR(S), 47382 47382 360 100.00 0.00 PERCUTANEOUS, RADIOFREQUENCY ABLATION, OPEN, OF ONE OR MORE LIVER 47381 47381 360 100.00 0.00 TUMOR(S); CRYOSURGICAL ABLATION, OPEN, OF ONE OR MORE LIVER 47380 47380 360 100.00 0.00 TUMOR(S); RADIOFREQUENCY ABRASION; EACH ADDITIONAL FOUR LESIONS OR LESS (LIST SEPARATELY IN ADDITION TO 15787 15787 360 100.00 0.00 CODE FOR PRIMARY PROCEDURE) ABRASION; SINGLE LESION (EG, KERATOSIS, 15786 15786 360 100.00 0.00 SCAR) ACETABULOPLASTY; (EG, WHITMAN, COLONNA, 27120 27120 360 100.00 0.00 HAYGROVES, OR CUP TYPE) ACETABULOPLASTY; RESECTION, FEMORAL 27122 27122 360 100.00 0.00 HEAD (EG, GIRDLESTONE PROCEDURE) ACETONE OTHER KETONE BODIES 82009 82009 301 60.00 100.00 60.00 ACROMIOPLASTY OR ACROMIONECTOMY, PARTIAL, WITH OR WITHOUT 23130 23130 360 17,250.00 100.00 17,250.00 CORACOACROMIAL LIGAMENT RELEASE ACTH 82024 82024 301 300.00 100.00 300.00 ACUTE HEPATITIS PANEL 80074 80074 300 1,210.00 100.00 1,210.00 ADAPT/EXT, PACING OR NEUROSTIMULATOR C1883-G C1883 278 700.00 0.00 LEAD IMPLANTABLE ADAPTER/EXT, PACING OR NEUROSTIMULATOR C1883-W C1883 278 100.00 0.00 LEAD IMPLANTABLE ADENOIDECTOMY, PRIMARY; AGE 12 OR OVER 42831 42831 360 8,900.00 100.00 8,900.00 ADENOIDECTOMY, PRIMARY; UNDER AGE 12 42830 42830 -

Imaging Shoulder Impingement
UCLA UCLA Previously Published Works Title Imaging shoulder impingement. Permalink https://escholarship.org/uc/item/0kg9j32r Journal Skeletal radiology, 22(8) ISSN 0364-2348 Authors Gold, RH Seeger, LL Yao, L Publication Date 1993-11-01 DOI 10.1007/bf00197135 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Skeletal Radiol (1993) 22:555-561 Skeletal Radiology Review Imaging shoulder impingement Richard H. Gold, M.D., Leannc L. Seeger, M.D., Lawrence Yao, M.D. Department of Radiological Sciences, UCLA School of Medicine, 10833 Le Conte Avenue, Los Angeles, CA 90024-1721, USA Abstract. Appropriate imaging and clinical examinations more components of the coracoacromial arch superiorly. may lead to early diagnosis and treatment of the shoulder The coracoacromial arch is composed of five basic struc- impingement syndrome, thus preventing progression to a tures: the distal clavicle, acromioclavicular joint, anteri- complete tear of the rotator cuff. In this article, we discuss or third of the acromion, coracoacromial ligament, and the anatomic and pathophysiologic bases of the syn- anterior third of the coracoid process (Fig. 1). Repetitive drome, and the rationale for certain imaging tests to eval- trauma leads to progressive edema and hemorrhage of uate it. Special radiographic projections to show the the rotator cuff and hypertrophy of the synovium and supraspinatus outlet and inferior surface of the anterior subsynovial fat of the subacromial bursa. In a vicious third of the acromion, combined with magnetic reso- cycle, the resultant loss of space predisposes the soft nance images, usually provide the most useful informa- tissues to further injury, with increased pain and disabili- tion regarding the causes of impingement. -
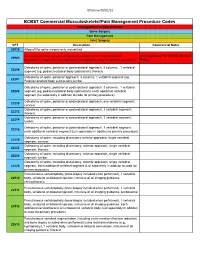
Commercial MSK Procedure Code List Effective 09.01.21.Xlsx
Effective 09/01/21 BCBST Commercial Musculoskeletal/Pain Management Procedure Codes Investigational or Non-Covered Spine Surgery Pain Management Joint Surgery CPT Description Commercial Notes 20930 Allograft for spine surgery only morselized Computer-assisted surgical navigational procedure for musculoskeletal Investigational Per BCBST Medical 20985 procedures, image-less (List separately in addition to code for primary procedure) Policy Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral 22206 segment (eg, pedicle/vertebral body subtraction); thoracic Osteotomy of spine, posterior approach, 3 columns, 1 vertebral segment (eg. 22207 Pedicle/vertebral body subtraction);lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral 22208 segment (eg, pedicle/vertebral body subtraction); each additional vertebral segment (list separately in addition to code for primary procedure) Osteotomy of spine, posterior or posterolateral approach, one vertebral segment; 22210 cervical Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22212 thoracic Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22214 lumbar Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22216 each additional vertebral segment (List separately in addition to primary procedure) Osteotomy of spine, including discectomy anterior approach, single vertebral 22220 segment; cervical Osteotomy of spine, including discectomy, anterior approach, single -

The Role of Acromioplasty for Rotator Cuff Problems
The Role of Acromioplasty for Rotator Cuff Problems Jonathan M. Frank, MDa,*, Jaskarndip Chahal, MD, FRCSCb, Rachel M. Frank, MDa, Brian J. Cole, MD, MBAc, Nikhil N. Verma, MDc, Anthony A. Romeo, MDc KEYWORDS Acromioplasty Subacromial decompression Impingement syndrome Rotator cuff repair KEY POINTS Acromioplasty is a well-described technique used for a variety of rotator cuff pathologies, with a rapid rise in its use over the past several years. There are 2 competing theories regarding rotator cuff pathology—intrinsic and extrinsic—that either support or limit the potential benefits of acromioplasty. Acromioplasty may be an effective treatment option for subacromial impingement refractory to conservative therapy. The utility of acromioplasty at the time of rotator cuff repair has come into question, with new studies showing no significant benefit. Further studies with long-term follow-up are required to determine the efficacy of acromioplasty for impingement syndrome and during rotator cuff repair. INTRODUCTION modifications have been proposed. For example, in 1987, Ellman7 described an arthroscopic tech- In 1972, Neer first described acromioplasty and re- nique to accomplish coracoacromial ligament ported on its utility in treating chronic impingement 1 release, resection of the anterior acromion under- syndrome. He postulated acromial morphology surface, and bursal de´ bridement, which he as the initiating factor leading to dysfunction of arthroscopic subacromial decompression 1,2 termed, the rotator cuff and eventual tearing. This tenet (SAD). McCallister and colleagues8 as well as Mat- is the basis for the extrinsic theory of rotator cuff sen and Lippitt9 described a “smooth and move,” degeneration and has had a profound impact on which involves an extensive bursectomy and surgical practice, with several investigators advo- smoothing of the undersurface of the acromion cating for concomitant acromioplasty during 3–6 without altering acromial morphology. -

Modified Arthroscopic Latarjet Procedure: Suture-Button Fixation Will Not
1 Modified Arthroscopic Latarjet Procedure: Suture-button Fixation will not 2 Cause Obvious Increasement of Superior Instability at 5-Year Follow-up 3 Daqiang Liang1, Haifeng Liu1,2,3,4,5, Xinzhi Liang1,2, Qihuang Qin1, Lujue Long6, 4 Yong Huang1,2, Wei Lu1,2,3,4,5*, Zhenhan Deng1,2,3,4,5* 5 1 Department of Sports Medicine, Key Laboratory of Tissue Engineering of Shenzhen, 6 Shenzhen Second People’s Hospital/ the First Affiliated Hospital of Shenzhen 7 University Health Science Center, Shenzhen 518035, Guangdong, China; 8 2 Clinical Medical College, Shenzhen University, Shenzhen 518000, Guangdong, 9 China; 10 3 Guangzhou Medical University, Guangzhou 510182, Guangdong, China; 11 4 Anhui Medical University, Hefei 230032, Anhui, China; 12 5 Guangxi University of Chinese Medicine, Nanning 530229, Guangxi, China; 13 6 Xiangya Stomatological Hospital & School of Stomatology, Central South 14 University, Changsha 410008, Hunan, China. 15 Daqiang Liang and Haifeng Liu contributed equally. 16 *Address correspondence to: Dr. Zhenhan Deng and Dr. Wei Lu, Department of 17 Sports Medicine, Shenzhen Second People’s Hospital/ 18 the First Affiliated Hospital of Shenzhen University Health Science Center, Shenzhen 19 410008, Guangdong, China. Tel: +86-13929440786 (ZHD) and +86-13922855513 20 (WL); Email: [email protected] (ZHD) and [email protected] 21 (WL) 22 Trial registration 23 Retrospectively registered. 1 24 Background: Whether coracoacromial ligament (CAL) release during Latarjet 25 procedure will increase superior instability of shoulder joint postoperatively remains 26 controversial. This study aims to observe changes in the acromiaohumeral distance 27 (AHD) of patients who underwent modified double-button Latarjet procedure and 28 provide evidence to address the issue. -

Open Latarjet: a Reliable, Successful Method to Prevent Recurrence in the Presence of Bony Defects Kevin D
Open Latarjet: A Reliable, Successful Method to Prevent Recurrence in the Presence of Bony Defects Kevin D. Plancher, MD,†,‡,§ Stephanie C. Petterson, PhD,† and Gilles Walch, MD* Glenoid bone loss may dictate the success of procedures to restore anterior shoulder instability. The Latarjet procedure addresses bony defects to minimize the risk of recurrence in this subset of patients with bone loss in both athletes and non-athletes alike. This article describes a modified, open Latarjet procedure using a subscapularis splitting technique that provides stability through the triple-blocking effect previously described by Patte et al. The “sling effect”, a dynamic effect created by the transfer of the conjoint tendon, provides stabilization in abducted and externally rotated arm positions particularly at mid and end ranges of motion. Augmentation of the anteroinferior glenoid increases or restores the glenoid diameter to provide stability through a “bone blocking effect”. Lastly, stability is achieved by repairing the capsule to the coracoacromial ligament stump. This open procedure has been utilized successfully when a physician is confronted by this difficult clinical scenario. Oper Tech Sports Med 21:238-245 C 2013 Elsevier Inc. All rights reserved. KEYWORDS shoulder, open Latarjet, anterior instability, bony defect he success of arthroscopic Bankart repair is dependent on acts as a sling on the inferior subscapularis and anteroinferior Tthe amount of glenoid boss loss. Studies have demon- capsule when the arm is abducted and externally rotated. strated that in the presence of a significant bone loss, defined as Second, the “bone blocking effect” by augmentation of the a bone loss of greater than 25%, recurrence rates are as high as anteroinferior glenoid increases or restores, the glenoid diameter 67% when failing to address this defect intraoperatively.1 We in the anteroposterior direction. -
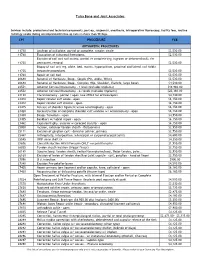
Tulsa Bone and Joint Associates CPT PROCEDURE
Tulsa Bone and Joint Associates Services include: professional and technical components, post-op, surgeon(s), anesthesia, intraoperative fluoroscopy, facility fees, routine radiology exams during uncomplicated follow-up care no more than 90 days. CPT PROCEDURE FEE ORTHOPEDIC PROCEDURES 11730 Avulsion of nail plate, partial or complete, simple; single $2,530.00 11740 Evacuation of subungual hematoma. $2,530.00 Excision of nail and nail matrix, partial or complete (eg, ingrown or deformed nail), for 11750 permanent removal $2,530.00 Biopsy of nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) 11755 (separate procedure). $2,530.00 11760 Repair of nail bed. $2,530.00 20680 Removal of Hardware, Deep - Simple (Pin, Ankle, Wrist) $2,530.00 20680 Removal of Hardware, Deep - Complex (Hip, Shoulder, Clavicle, large bone) $4,510.00 22551 Anterior Cervical Discectomy - 1 level (includes implants) $18,960.00 22552 Anterior Cervical Discectomy - 2+ levels (includes implants) $25,360.00 23120 Claviculectomy - partial / open (use 29824 for arthroscopic) $4,730.00 23410 Repair rotator cuff acute - open $6,150.00 23412 Repair rotator cuff chronic - open $6,150.00 23415 Release of shoulder ligament w/wo acromioplasty - open $6,150.00 23420 Reconstruction of complete shoulder cuff auvlsion w/ acromioplasty - open $6,150.00 23430 Biceps Tenodesis - open $4,950.00 23455 Bankhart w/ labral repair - open $6,150.00 23462 Capsulorrhaphy, anterior w coracoid transfer - open $6,150.00 25000 Incision, extensor tendon sheath - DeQuervains -

Physicians As Assistants at Surgery: 2016 Update
Physicians as Assistants at Surgery: 2016 Update Participating Organizations: American College of Surgeons American Academy of Ophthalmology American Academy of Orthopaedic Surgeons American Academy of Otolaryngology – Head and Neck Surgery American Association of Neurological Surgeons American Pediatric Surgical Association American Society of Colon and Rectal Surgeons American Society of Plastic Surgeons American Society of Transplant Surgeons American Urological Association Congress of Neurological Surgeons Society for Surgical Oncology Society for Vascular Surgery Society of American Gastrointestinal Endoscopic Surgeons The American College of Obstetricians and Gynecologists The Society of Thoracic Surgeons Physicians as Assistants at Surgery: 2016 Update INTRODUCTION This is the seventh edition of Physicians as Assistants at Surgery, a study first undertaken in 1994 by the American College of Surgeons and other surgical specialty organizations. The study reviews all procedures listed in the “Surgery” section of the 2016 American Medical Association’s Current Procedural Terminology (CPT TM). Each organization was asked to review new codes since 2013 that are applicable to their specialty and determine whether the operation requires the use of a physician as an assistant at surgery: (1) almost always; (2) almost never; or (3) some of the time. The results of this study are presented in the accompanying report, which is in a table format. This table presents information about the need for a physician as an assistant at surgery. Also, please note that an indication that a physician would “almost never” be needed to assist at surgery for some procedures does NOT imply that a physician is never needed. The decision to request that a physician assist at surgery remains the responsibility of the primary surgeon and, when necessary, should be a payable service. -

Priority Health Spine and Joint Code List
Priority Health: Joint Services CPT Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER