Phase III, Randomized, Placebo-Controlled, Double-Blind
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nilotinib in Patients with Chronic Myeloid Leukemia: STAT2 Trial in Japan
Haematologica HAEMATOL/2018/194894 Version 3 Haematologica HAEMATOL/2018/194894 Version 3 Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan Naoto Takahashi, Kaichi Nishiwaki, Chiaki Nakaseko, Nobuyuki Aotsuka, Koji Sano, Chikako Ohwada, Jun Kuroki, Hideo Kimura, Michihide Tokuhira, Kinuko Mitani, Kazuhisa Fujikawa, Osamu Iwase, Kohshi Ohishi, Fumihiko Kimura, Tetsuya Fukuda, Sakae Tanosaki, Saori Takahashi, Yoshihiro Kameoka, Hiroyoshi Nishikawa, and Hisashi Wakita Disclosures: 1. This study was supported by research funding from Novartis Pharmaceuticals to N.T. 2. N.T reports grants from Novartis Pharmaceuticals, during the conduct of the study; grants and personal fees from Novartis Pharmaceuticals, grants and personal fees from Otsuka, grants and personal fees from Pfizer, personal fees from Bristol-Myers Squibb, outside the submitted work; K.N reports grants from Zenyaku Kogyo Company, Limited, grants from Chugai Pharmaceutical, grants from Novartis Pharma K.K., grants from Kyowa Hakko Kirin Co, Ltd, grants from Nippon Shinyaku Co, Ltd, outside the submitted work; C.N reports personal fees from Novartis, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Pfizer, grants and personal fees from Takeda pharmaceuticals, grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Otsuka Pharmaceutical, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Asahi Kasei Pharma, grants and personal fees from Shionogi, personal fees from Shire, personal fees from Jannsen, personal fees from Celgene, outside the submitted work; M.T. reports personal fees from Bristol-Myers Squib, personal fees from Pfizer, outside the submitted work; K.M reports grants from Kyowa Hakko Kirin Co. -

Focus on Membrane Technology for Water Treatment
FocusFocus onon MembraneMembrane TechnologyTechnology forfor WaterWater TreatmentTreatment Toray Industries, Inc. February 4, 2004 Masaru Kurihara CONTENTCONTENT 1.1. WorldWorld WaterWater ProblemProblem 2.2. WaterWater TreatmentTreatment MembranesMembranes 3.3. RORO MembranesMembranes && NFNF MembranesMembranes 4.4. UFUF MembranesMembranes && MFMF MembranesMembranes -- DrinkingDrinking WaterWater ProductionProduction -- 5.5. ImmersedImmersed MembranesMembranes forfor WastewaterWastewater TreatmentTreatment 6.6. ConclusionConclusion TorayToray –– TheThe LeaderLeader inin ““AdvancedAdvanced MaterialsMaterials”” AchievingAchieving HighHigh GrowthGrowth byby ConstantlyConstantly SupplyingSupplying “Advanced“Advanced Materials”Materials”––DDevelopedeveloped withwith ourour CoreCore TechnologiesTechnologies ––iintonto ourour ThreeThree GrowthGrowth AreasAreas (an(an expansionexpansion ofof ourour fourfour strategicstrategic businessbusiness areas)areas) <Advanced Materials> •Nanofibers Four Strategic •High-performance Fibers <Three Growth Areas> Business Areas <Core Technologies> and Resins Electronics & Info- IT-related Products Organic Synthetic •Nano-alloy Materials Related Products Chemistry •Advanced Electronics Materials Life Sciences Pharmaceuticals Polymer Chemistry •Biomaterials •Separation Materials Water Treatment Biochemistry •High-performance Composite Environment Safety Materials Amenity •Recycling Materials Carbon Fiber WorldWorld WaterWater ShortageShortage -- NowNow andand FutureFuture (WMO and others, 1996) 1995 Main regions -

Expansion Strategy of Water Treatment Business 19Th December 2008
<5th IT-2010 IR Seminar> Expansion Strategy of Water Treatment Business 19th December 2008 Toray Industries, Inc Executive Vice President and General Manager of Water Treatment & Environment Div. Akihiro Nikkaku General Manager of Water Treatment Div. Hideo Sato 1 Contents: 1.Global Water Environment and Toray’s Activity 2. Toray’s Water Treatment Business 3. Reverse Osmosis (RO) Membrane Business 4. Submerged Membrane Business for Membrane Bioreactor (MBR) 5. Hollow Fiber UF/MF Membrane Business 6. IMS (Integrated Membrane System) 7. Expansion Plan of Water Treatment Business 2 Global Environmental Issues: Co2 and Water Water Issue (Water Shortage and Water CO2 Issue Pollution) Acid Rain Desertification Diminishing Global Warming Rain Forest Pollution Issues Ocean Pollution Destruction of Waste Problem Ozone Layer 3 Toray’s Approach toward Global Water Environment Issue Global Environmental Issue: Water became a focus at World Economic Forum 2007 (Commonly known as:Davos Conference) Jan 25,2007,Davos: Toray co-sponsored Special Japanese Sushi Reception 2007 , which was hosted by Japan Water Forum (JWF) The 1st Asia Pacific Water Summit (Beppu, Japan): Organizer: APWF(JWF) Dec.3-4, 2007, Beppu: Toray attended at CEO PANEL and made the presentation titled “Membrane Technologies meet to the Solution of the Subjects on the Global Water Environment “ Toray attended the Liberal Democratic Party research group on water security Mar.23, 2008, Toray made a report on “Approach to Rapidly Growing Global Water Business Market” as one of the major members of Council on Competitiveness- Nippon (COCN)’s “Technologies for Effective Utilization of Water Treatment and Water Resource Project” Toray participated in Singapore International Water Week June 23-27, 2008,Singapore:Toray gave a keynote speech at Japan Business Forum. -

Factset-Top Ten-0521.Xlsm
Pax International Sustainable Economy Fund USD 7/31/2021 Port. Ending Market Value Portfolio Weight ASML Holding NV 34,391,879.94 4.3 Roche Holding Ltd 28,162,840.25 3.5 Novo Nordisk A/S Class B 17,719,993.74 2.2 SAP SE 17,154,858.23 2.1 AstraZeneca PLC 15,759,939.73 2.0 Unilever PLC 13,234,315.16 1.7 Commonwealth Bank of Australia 13,046,820.57 1.6 L'Oreal SA 10,415,009.32 1.3 Schneider Electric SE 10,269,506.68 1.3 GlaxoSmithKline plc 9,942,271.59 1.2 Allianz SE 9,890,811.85 1.2 Hong Kong Exchanges & Clearing Ltd. 9,477,680.83 1.2 Lonza Group AG 9,369,993.95 1.2 RELX PLC 9,269,729.12 1.2 BNP Paribas SA Class A 8,824,299.39 1.1 Takeda Pharmaceutical Co. Ltd. 8,557,780.88 1.1 Air Liquide SA 8,445,618.28 1.1 KDDI Corporation 7,560,223.63 0.9 Recruit Holdings Co., Ltd. 7,424,282.72 0.9 HOYA CORPORATION 7,295,471.27 0.9 ABB Ltd. 7,293,350.84 0.9 BASF SE 7,257,816.71 0.9 Tokyo Electron Ltd. 7,049,583.59 0.9 Munich Reinsurance Company 7,019,776.96 0.9 ASSA ABLOY AB Class B 6,982,707.69 0.9 Vestas Wind Systems A/S 6,965,518.08 0.9 Merck KGaA 6,868,081.50 0.9 Iberdrola SA 6,581,084.07 0.8 Compagnie Generale des Etablissements Michelin SCA 6,555,056.14 0.8 Straumann Holding AG 6,480,282.66 0.8 Atlas Copco AB Class B 6,194,910.19 0.8 Deutsche Boerse AG 6,186,305.10 0.8 UPM-Kymmene Oyj 5,956,283.07 0.7 Deutsche Post AG 5,851,177.11 0.7 Enel SpA 5,808,234.13 0.7 AXA SA 5,790,969.55 0.7 Nintendo Co., Ltd. -

Research and Development in Breast Ultrasound E
E. Ueno, T. Shiina, M. Kubota, K. Sawai (Eds.) Research and Development in Breast Ultrasound E. Ueno, T. Shiina M. Kubota, K. Sawai (Eds.) Research and Development in Breast Ultrasound With 150 Figures, Including 37 in Color 1 3 Ei Ueno, M.D., Ph.D. Associate Professor, Department of Breast-Thyroid-Endocrine Surgery Institute of Clinical Medicine, University of Tsukuba 1-1-1 Tennodai, Tsukuba, Ibaraki 304-8573, Japan Tsuyoshi Shiina, Ph.D. Professor, Institute of Information Sciences and Electronics, University of Tsukuba 1-1-1 Tennodai, Tsukuba, Ibaraki 304-8573, Japan Mitsuhiro Kubota, M.D. Director of Yamachika Memorial Hospital 3-19-14 Koyawata, Odawara, Kanagawa 256-0815, Japan Kiyoshi Sawai, M.D. Associate Professor, Department of Endocrine & Breast Surgery Kyoto Prefectural University of Medicine 465 Kajii-cho, Kawaramachi-Hirokoji, Kamigyo-ku, Kyoto 602-0841, Japan Cover Illustration: Madoka Momota Library of Congress Control Number: 2004116202 ISBN 4-431-40277-2 Springer-Verlag Tokyo Berlin Heidelberg New York This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broad- casting, reproduction on microfilms or in other ways, and storage in data banks. The use of registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Product liability: The publisher can give no guarantee for information about drug dosage and appli- cation thereof contained in this book. -

Auction Held September 21, 2019. Partial List of Equipment & Vehicles
Auction held September 21, 2019. Partial list of Equipment & Vehicles: Unit # Description Serial # MN2568 19 INCH FLAT PANEL MONITOR HP L1910 CNC932P6R2 MN2545 19 INCH FLAT PANEL MONITOR HP L1910 CNC921Q2D6 MN2574 19 INCH FLAT PANEL MONITOR HP L1910 CNC932P7PB 3387 1998 INTERNATIONAL 1HTSMABK1WH515913 31088 2004 CHEV ASTRO VAN 1GNEL19X04B128713 31006 2004 FORD F-250 PICKUP 1FTNX21L04ED47326 31097 2005 FORD F-250 1FTSX21505ED07947 31097 2005 KNAPHEIDE 8' SERVICE BODY 696D38J 33080 2006 INTERNATIONAL 10 WHEEL TRUCK 1HTWYAHT96J360476 31128 2008 GMC CANYON 4WD REG CAB 1GTDT14EX88166799 36910 2008 GMC SIERRA 3500 HD 4WD EX 1GTHK29K78E157682 33520 2009 GMC CANYON 4WD 1GTDT19EX98148913 31149 2009 GMC CANYON 4WD EXT CAB 1GTDT19E198147004 31182 2011 GMC CANYON 2WD EXT CAB 1GTC5MFE8B8112137 261468 AERIAL LIFT NA 10023 BACKHOE JJ00247668 52087 BMW POLICE MOTORCYCLE WB104400XCZW21447 52088 BMW POLICE MOTORCYCLE WB1044008CZW21446 52089 BMW POLICE MOTORCYCLE WB1044006CZW21445 52090 BMW POLICE MOTORCYCLE WB1044004CZW21444 52092 BMW POLICE MOTORCYCLE WB1044005CZW21436 52094 BMW POLICE MOTORCYCLE WB1044001CZW21434 52095 BMW POLICE MOTORCYCLE WB104400XCZW21433 52096 BMW POLICE MOTORCYCLE WB1044000CZW21618 24005 BOBTAIL DUMP TRUCK 1HTSDAAR91H354251 40480 CHEV IMPALA 2G1WS583789245100 40481 CHEV IMPALA 2G1WS583689249042 40491 CHEV IMPALA 2G1WS583789274774 44030 CHEV IMPALA 2G1WS58R979297119 44054 CHEV IMPALA 2G1WD5EMXA1153507 50268 CHEV IMPALA 2G1WS58R879234724 50278 CHEV IMPALA 2G1WS58R479272595 50298 CHEV IMPALA 2G1WS583089239543 50307 CHEV IMPALA 2G1WS583889236907 -

Changes of Directors and Executive Officers
March 22, 2021 Mizuho Financial Group, Inc. Changes of Directors and Executive Officers Mizuho Financial Group, Inc. hereby announces changes of Member of the Board of Directors and Executive Officers (including changes in their areas of responsibility, etc.) of the following entities within the Group : Mizuho Financial Group, Inc. (MHFG) Mizuho Bank, Ltd. (MHBK) Mizuho Trust & Banking Co., Ltd. (MHTB) Mizuho Securities Co., Ltd. (MHSC) Mizuho Research & Technologies, Ltd. (MHRT) 1 Contents Mizuho Financial Group, Inc. (MHFG) .......................................................................................................... 3 1. Changes of Member of the Board of Directors ..................................................................................... 3 2. Changes of Executive Officers .............................................................................................................. 3 3. Directors and Executive Officers as of April 1, 2021 .......................................................................... 5 Mizuho Bank, Ltd. (MHBK) ............................................................................................................................ 8 1. Changes of Directors and Executive Officers ...................................................................................... 8 2. Directors and Executive Officers as of April 1, 2021 ......................................................................... 12 Mizuho Trust & Banking Co., Ltd. (MHTB) ............................................................................................... -
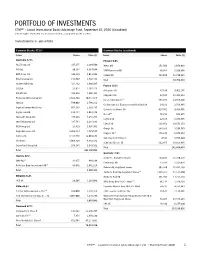
Portfolio of Investments
PORTFOLIO OF INVESTMENTS CTIVP® – Lazard International Equity Advantage Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.6% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 6.9% Finland 1.0% AGL Energy Ltd. 437,255 4,269,500 Metso OYJ 153,708 2,078,669 ASX Ltd. 80,181 4,687,834 UPM-Kymmene OYJ 36,364 1,106,808 BHP Group Ltd. 349,229 9,021,842 Valmet OYJ 469,080 11,570,861 Breville Group Ltd. 153,867 2,792,438 Total 14,756,338 Charter Hall Group 424,482 3,808,865 France 9.5% CSL Ltd. 21,611 4,464,114 Air Liquide SA 47,014 7,452,175 Data#3 Ltd. 392,648 1,866,463 Capgemini SE 88,945 11,411,232 Fortescue Metals Group Ltd. 2,622,808 30,812,817 Cie de Saint-Gobain(a) 595,105 24,927,266 IGO Ltd. 596,008 1,796,212 Cie Generale des Etablissements Michelin CSA 24,191 2,596,845 Ingenia Communities Group 665,283 2,191,435 Electricite de France SA 417,761 4,413,001 Kogan.com Ltd. 138,444 2,021,176 Elis SA(a) 76,713 968,415 Netwealth Group Ltd. 477,201 5,254,788 Legrand SA 22,398 1,783,985 Omni Bridgeway Ltd. 435,744 1,234,193 L’Oreal SA 119,452 38,873,153 REA Group Ltd. 23,810 1,895,961 Orange SA 298,281 3,106,763 Regis Resources Ltd. -

Factors Associated with Non-Remission in Bipolar Disorder: the Multicenter Treatment Survey for Bipolar Disorder in Psychiatric Outpatient Clinics (MUSUBI)
Neuropsychiatric Disease and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Factors Associated with Non-Remission in Bipolar Disorder: The Multicenter Treatment Survey for Bipolar Disorder in Psychiatric Outpatient Clinics (MUSUBI) This article was published in the following Dove Press journal: Neuropsychiatric Disease and Treatment Takashi Tsuboi, 1,2 Purpose: The aim of this study was to identify factors associated with non-remission in 2,3 Ta kefum i Suzuk i, bipolar disorder. 4 Takaharu Azekawa, Patients and Methods: The multicenter treatment survey for bipolar disorder in psychia- 4 4 Naoto Adachi, Hitoshi Ueda, tric outpatient clinics (MUSUBI) study used a questionnaire administered at 176 clinics 4 Kouji Edagawa, throughout Japan from September to October 2016. Clinic psychiatrists performed Eiichi Katsumoto,4 a retrospective medical record survey of consecutive cases with bipolar disorder. Patients Yukihisa Kubota, 4 were considered to be in remission if they met all of the following criteria: they were not in Eiichiro Goto,4 Seiji Hongo,4 4 a mixed state, their manic or depressive symptoms were either borderline or nonexistent Yoichiro Watanabe, Masaki Kato, 2,5 Norio (corresponding to 2 or 1 points on the Clinical Global Impressions Scale, Bipolar Version), Yasui-Furukori,2,6 and their psychiatrists clinically considered them to be in remission. Enrolled patients were Reiji Yoshimura,2,7 classified into remitters group and non-remitters group and demographic and clinical char- Atsuo Nakagawa, 2,8 acteristics were contrasted between the groups. Non-remitters were compared with remitters, Toshiaki Kikuchi,2,8 using a series of logistic regression analyses. -

Machine Tool Business Operations
Briefing session on Air-conditioning & Refrigeration Systems, Paper & Printing Machinery and Machine Tool operations Section 3: Machine Tool Operations September 27, 2007 Ken Watabe Senior Vice President General Manager, Machine Tool Division All copyrights and intellectual property relating to this document belong to Mitsubishi Heavy Industries Ltd. Positioning of machine tool operations Shipbuilding Others & Ocean Mass and Others Aerospace Medium-Lot Manufactured Paper and Machine Machinery Printing Tools Machinery Machinery & Steel S tructures Power S ystems Industrial Machinery Net sales in 2006 Net sales in 2006 (consolidated) (consolidated) ¥219.3 billion Approx. 30% (Figure for the entire (Figure for Industrial company: ¥3,068.5 billion) Machinery: ¥219.3 billion) Other areas: food packaging machinery, injection molding machinery, industrial washing machinery, etc. All copyrights and intellectual property relating to this document belong to Mitsubishi Heavy Industries Ltd. 1 A diverse range of products in line with users’ needs Micro milling machines Special-purpose Gear cutting machines machines Large machines Machining centers Processing bodies and Cylinder blocks/ other large dies/molds mission cases Processing sensors and Molds for plastic other μ components components Transmission Gears for various electrically driven devices Transmission parts Room temperature wafer bonding machines Engine valves Power transmissions Precision cutting tools All copyrights and intellectual property relating to this document belong to Mitsubishi -
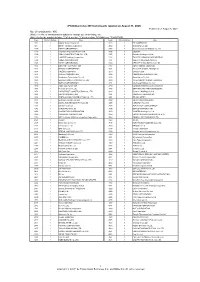
JPX-Nikkei Index 400 Constituents (Applied on August 31, 2021) Published on August 6, 2021 No
JPX-Nikkei Index 400 Constituents (applied on August 31, 2021) Published on August 6, 2021 No. of constituents : 400 (Note) The No. of constituents is subject to change due to de-listing. etc. (Note) As for the market division, "1"=1st section, "2"=2nd section, "M"=Mothers, "J"=JASDAQ. Code Market Divison Issue Code Market Divison Issue 1332 1 Nippon Suisan Kaisha,Ltd. 3048 1 BIC CAMERA INC. 1417 1 MIRAIT Holdings Corporation 3064 1 MonotaRO Co.,Ltd. 1605 1 INPEX CORPORATION 3088 1 Matsumotokiyoshi Holdings Co.,Ltd. 1719 1 HAZAMA ANDO CORPORATION 3092 1 ZOZO,Inc. 1720 1 TOKYU CONSTRUCTION CO., LTD. 3107 1 Daiwabo Holdings Co.,Ltd. 1721 1 COMSYS Holdings Corporation 3116 1 TOYOTA BOSHOKU CORPORATION 1766 1 TOKEN CORPORATION 3141 1 WELCIA HOLDINGS CO.,LTD. 1801 1 TAISEI CORPORATION 3148 1 CREATE SD HOLDINGS CO.,LTD. 1802 1 OBAYASHI CORPORATION 3167 1 TOKAI Holdings Corporation 1803 1 SHIMIZU CORPORATION 3231 1 Nomura Real Estate Holdings,Inc. 1808 1 HASEKO Corporation 3244 1 Samty Co.,Ltd. 1812 1 KAJIMA CORPORATION 3254 1 PRESSANCE CORPORATION 1820 1 Nishimatsu Construction Co.,Ltd. 3288 1 Open House Co.,Ltd. 1821 1 Sumitomo Mitsui Construction Co., Ltd. 3289 1 Tokyu Fudosan Holdings Corporation 1824 1 MAEDA CORPORATION 3291 1 Iida Group Holdings Co.,Ltd. 1860 1 TODA CORPORATION 3349 1 COSMOS Pharmaceutical Corporation 1861 1 Kumagai Gumi Co.,Ltd. 3360 1 SHIP HEALTHCARE HOLDINGS,INC. 1878 1 DAITO TRUST CONSTRUCTION CO.,LTD. 3382 1 Seven & I Holdings Co.,Ltd. 1881 1 NIPPO CORPORATION 3391 1 TSURUHA HOLDINGS INC. 1893 1 PENTA-OCEAN CONSTRUCTION CO.,LTD. -

TOBAM Maximum Diversification All World Developed Ex North America USD
TOBAM Maximum Diversification All World Developed ex North America USD 31/12/2019 Instrument Weight BP PLC 0.10% IDEMITSU KOSAN CO LTD 0.21% INPEX HOLDINGS INC 0.07% JX HOLDINGS INC 0.09% NESTE OIL OYJ 1.16% OMV AG 0.08% SANTOS LTD 0.02% SBM OFFSHORE NV 0.05% TGS NOPEC GEOPHYSICAL CO ASA 0.02% VOPAK 0.02% WOOD GROUP (JOHN) PLC 0.02% AIR LIQUIDE 0.23% AIR WATER INC 0.02% AKZO NOBEL 0.12% ALUMINA LTD 0.03% AMCOR PLC-CDI 0.08% AVON RESOURCES LTD 0.53% BORAL LTD 0.02% CHR HANSEN HOLDING A/S 0.08% DAICEL CHEMICAL INDUSTRIES 0.02% DOWA HOLDINGS CO LTD 0.01% EMS-CHEMIE HOLDING AG-REG 0.03% FLETCHER BUILDING LTD 0.02% FORTESCUE METALS GROUP LTD 0.60% GIVAUDAN-REG 0.16% HITACHI CHEMICAL CO LTD 0.03% HUHTAMAKI OYJ 0.03% ISRAEL CHEMICALS LTD 0.02% JAMES HARDIE INDUSTRIES-CDI 0.07% JFE HOLDINGS INC 0.02% KANSAI PAINT CO LTD 0.03% KURARAY CO LTD 0.03% MITSUBISHI MATERIALS CORP 0.02% NEWCREST MINING LTD 1.35% TOBAM Maximum Diversification All World Developed ex North America USD 31/12/2019 Instrument Weight NIPPON PAINT CO LTD 0.05% NIPPON PAPER INDUSTRIES CO L 0.04% NIPPON SHOKUBAI CO LTD 0.01% NISSAN CHEMICAL INDUSTRIES 0.04% NOF CORP 0.02% NORTHERN STAR RESOURCES LTD 0.66% NOVOZYMES A/S-B SHARES 0.07% OJI PAPER CO LTD 0.03% ORICA LTD 0.02% ORORA LTD 0.02% SARACEN MINERAL HOLDINGS LTD 0.32% SMURFIT KAPPA GROUP PLC 0.04% SYMRISE AG 0.04% TAIHEIYO CEMENT CORP 0.02% TAIYO NIPPON SANSO CORP 0.02% TEIJIN LTD 0.02% THYSSENKRUPP AG 0.04% TORAY INDUSTRIES INC 0.02% WIENERBERGER AG 0.02% ADP 0.04% AENA SA 0.09% ALFA LAVAL AB 0.04% ALL NIPPON AIRWAYS CO LTD