Geochemical Evaluation of Groundwater Quality in Selected Areas of Hyderabad, A.P., India G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Sq.Mt) No of Kits Madan Kumar Karana
Name of the house owner Area available on No of S.No House number & Location Sri/Smt terrace (Sq.mt) kits Flat No:101,Plot No: 28, Sri Lakshmi Nilayam, Krishna 100 Sq.mt 1 Madan Kumar Karanam 1 kit Nagar colony, Near Gandhian School, Picket, Sec’bad 4th floor 2 K.Venkateshwar 4-7-12/46A,Macharam, Ravindranagar, Hyd 400 sft 1 kit No:102, Bhargav residency, Enadu colony, 3 N.V.Krishna Reddy 2500 sft 4th floor 1kit Kukatpally,Hyderabad 500 sft 4 Ghous Mohiuddin 5-6-180,Aghapura, Hyd 1 kit 2nd floor 5 Abdul Wahed 18-1-350/73,Yousuf bin colony,chandrayangutta,Hyd 1 kit 260, Road no: 9B,Alkapuri, 6 Cherukupalli Narasimha Rao 1200 sft 1 kit Near sai baba temple 10-5-112,Ahmed Nagar, Masab tank, 7 Ahmed Nizamuzzana Quraishi Rs.3000 sft 1 kit Hymayunangar,yderabad 9-7-121/1, Maruthi nagar, Opp:Santhosh Nagar 8 K.Vjai Kumar 100 sft 1 kit colony Saidabad, Hyd 9 B. Sugunakar 8-2-121, Behind Big Bazar, Punjagutta, Hyd 900 sft 1 kit 10 D. Narasimha Reddy 3.33.33 LV Reddi colony, Lingampally, Hyd 900 sft 1 kit 1-25-176/9/1, Rahul enclave, Shiva nagar, 11 D. Radhika 1000 sft 1 kit Kanajiguda, Trimalgherry, Secunderabad 2-3-800/5, Plot no: D-4,Road no:15,Co-op bank 12 B. Muralidhara Gupta 1600 sft 1 kit colony, Nagole, Hyderabad 13 Dr.Mazar Ali 12-2-334/B,Murad nagar, Mehdipatnam,Hyderabad 1600 sft 2 kits 14 Sukhavasi Tejorani 22-32/1, VV nagar, Dilshukhnagar, Hyd 800 sft 1 kit 15 Induri Bhaskara Reddy MIG 664, Phase I & II, KPHB colony,Kukatpally, Hyd 1000 sft 1 kit No-18, Subhodaya nagar colony, near HUDA park, 16 T.Sundary 150 yards 1 kit Opp: KPHB, Kukatpally, Hyd. -
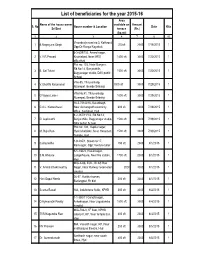
List of Beneficiaries for the Year 2015-16 Area Name of the House Owner Available on Amount S
List of beneficiaries for the year 2015-16 Area Name of the house owner available on Amount S. No House number & Location Date Kits Sri/Smt terrace (Rs.) (Sq.mt) 1 2 3 4 7 8 10 Viswabeeja road no:3, Kothapet, 1 B.Nagarjuna Singh 200sft 3000 7/15/2015 1 Opp:Dr.Ranga Nayakulu 6-3-609/153, Anand nagar, 2 V.V.R.Prasad Khairtabad, Near MRO 1400 sft 3000 7/20/2015 1 officeHyd Flat no: 103, Hotel Banjara, Rd.No:14, Banjarahills, 3 B. Sai Tulasi 1500 sft 3000 7/20/2015 1 Bagyanagar studio, DAV public School Villa-55, Thirusankalp, 4 V.Shanthi Karunamai 1000 sft 3000 7/29/2015 1 Nizampet, Beside Sribalaji Villa No:41, Thirusankalp, 5 G.Vijaya Laxmi 1000 sft 3000 7/29/2015 1 Nizampet, Beside Sribalaji 16-2-751/A/16, Karanbagh, 6 C.A.L. Kameshwari Near Asmangadh electricity 600 sft 3000 7/29/2015 1 office, Saidabad, Hyd 8-2-287/11/1/2, Rd.No:14, 7 D. Leelavathi Banjarahills, Bagyanagar studio, 1500 sft 3000 7/29/2015 1 DAV public School Plot no: 104, Kapila nagar, 8 M. Raja Rao Hydershahkote, Near Hanuman 1500 sft 3000 7/30/2015 1 Temple, Hyd 1-9-235/1, Street no:17, 9 C.Anuradha 150 sft 2000 8/1/2015 1 Ramnagar, Opp: Venture tailor 9-1-1/66/2, Hasimnagar, 10 S.M.Ghouse Langerhouse, Near fire station, 1700 sft 2000 8/1/2015 1 Hyd MIG-A-66, ECIL, Dr.AS Rao 11 V. -
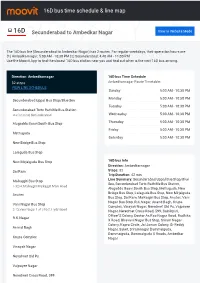
16D Bus Time Schedule & Line Route
16D bus time schedule & line map 16D Secunderabad to Ambedkar Nagar View In Website Mode The 16D bus line (Secunderabad to Ambedkar Nagar) has 2 routes. For regular weekdays, their operation hours are: (1) Ambedkarnagar: 5:00 AM - 10:30 PM (2) Secunderabad: 4:40 AM - 11:00 PM Use the Moovit App to ƒnd the closest 16D bus station near you and ƒnd out when is the next 16D bus arriving. Direction: Ambedkarnagar 16D bus Time Schedule 32 stops Ambedkarnagar Route Timetable: VIEW LINE SCHEDULE Sunday 5:00 AM - 10:30 PM Monday 5:00 AM - 10:30 PM Secunderabad Uppal Bus Stop/Blue Sea Tuesday 5:00 AM - 10:30 PM Secunderabad Tsrtc Rathiƒle Bus Station station road, Secunderabad Wednesday 5:00 AM - 10:30 PM Alugadda Baavi South Bus Stop Thursday 5:00 AM - 10:30 PM Friday 5:00 AM - 10:30 PM Mettuguda Saturday 5:00 AM - 10:30 PM New Bridge Bus Stop Lalaguda Bus Stop New Mirjalguda Bus Stop 16D bus Info Direction: Ambedkarnagar Sai Ram Stops: 32 Trip Duration: 42 min Malkajgiri Bus Stop Line Summary: Secunderabad Uppal Bus Stop/Blue Sea, Secunderabad Tsrtc Rathiƒle Bus Station, 5-32/4, Malkajgiri Malkajgiri Main Road Alugadda Baavi South Bus Stop, Mettuguda, New Bridge Bus Stop, Lalaguda Bus Stop, New Mirjalguda Anutex Bus Stop, Sai Ram, Malkajgiri Bus Stop, Anutex, Vani Nagar Bus Stop, R.K.Nagar, Anand Bagh, Krupa Vani Nagar Bus Stop Complex, Vinayak Nagar, Neredmet Old Ps, Vajpayee 2-73,Vani Nagar 1 st street, Hyderābād Nagar, Neredmet Cross Road, 599, Sainikpuri, O∆cer’S Colony, Doctor As Rao Nagar Road, Radhika R.K.Nagar X Road, Bhavani Nagar Bus Stop, Sriram Nagar Colony, Kapra Circle, Jai Jawan Colony, Gr Reddy Anand Bagh Nagar, Saket, Sriramnagar Dammaiguda, Dammaiguda, Dammaiguda X Roads, Ambedkar Krupa Complex Nagar Vinayak Nagar Neredmet Old Ps Vajpayee Nagar Neredmet Cross Road, 599 Sainikpuri O∆cer’S Colony Doctor As Rao Nagar Road Radhika X Road Dr A.S.Rao Nagar Road;Dr. -

Telangana State Information Commission
TELANGANA STATE INFORMATION COMMISSION (Under Right to Information Act, 2005) Samachara Hakku Bhavan, D.No.5-4-399, ‘4’ Storied Commercial Complex, Housing Board Building, Mojam Jahi Market, Hyderabad – 500 001. Phone Nos: 040-24740665 (o); 040-24740592(f) Appeal No.15604/SIC-KSR/2019 Dated: 15-12-2020 Appellant : Sri S. Anji Reddy, P.O ECIL Hyderabad-500 062. Respondents : The Public Information Officer (U/RTI Act, 2005) / O/o the Deputy Commissioner, Greater Hyderabad Municipal Corporation, Kapra, Circle-1, Hyderabad The Appellate Authority (U/RTI Act, 2005) / O/o the Zonal Commissioner, Greater Hyderabad Municipal Corporation, LB Nagar Zone, Gaddi Annaram, Hyderabad-500 060. O R D E R Sri S. Anji Reddy, P.O ECIL Hyderabad-500 062 has filed 2nd appeal dated 18-12-2019 which was received by this Commission on 27-12-2019 for not getting the information sought by him from the PIO / O/o the Deputy Commissioner, Greater Hyderabad Municipal Corporation, Kapra, Circle-1, Hyderabad and 1st Appellate Authority / O/o the Zonal Commissioner, Greater Hyderabad Municipal Corporation, LB Nagar Zone, Gaddi Annaram, Hyderabad-500 060. The brief facts of the case as per the appeal and other records received along with it are that the appellant herein filed an application dated 05-10-2019 before the PIO under Sec.6(1) of the RTI Act, 2005, requesting to furnish the information on the following points mentioned in his application: TSIC The Public Information Officer has not furnished the information to the appellant. Since the appellant did not receive the information from the Public Information Officer, he filed 1st appeal dated 17-11-2019 before the 1st Appellate Authority u/s 19(1) of the RTI Act, 2005 requesting him to furnish the information sought by him. -

1. Agriculture
1. AGRICULTURE • Agriculture sector plays a pivotal role in meeting the dietary requirements of one of the most densely populated districts - Medchal – Malkajgiri. • Total Agriculture land in the District (as per RB record) : 70000 Ac Net cultivated area : 26807 Ac • Predominant crops: Paddy (47% of cultivated area), Maize (11%), Fodder (6%), Redgram (3%), Cotton (2%), Other food crops (30%) of cultivated area. • Soils : Red soil is predominant (70%), Black soils (30%) • Source of irrigation: Bore wells : 14000 Ac Minor irrigation tanks: 2600 Ac Musi : 3000 Ac • Rainfall Normal :711 mm Actual: 813.7mm % Deviation: 14 Normal No .of Rainy Days : 59 • Market facility : Through procurement centres, AMC Medchal, Pvt traders, Local markets • Agri Input supply : Subsidy seed is distributed to farmers through online portal (OSSDS) at 7 notified sale points (3PACS, 3FACS, 1 MGC), Major sales are through private dealers(22) • Fertilizer supply through PACS, FSCS, Pvt dealers (42). • Rythu Samanvaya Samithies: 101 GRSS, 6MRSS 1ZRSS: Total 108 Flagship programmes / Schemes implemented during the Yr. 2019-20 Sl. Achievem % Scheme Target Remarks No ent Ach I FLAG SHIP PROG. Bank details of 27451farmers (except give it up, not 33334 23951 Far 71% interested cases) updated and Rythu Bandhu 1 Farmers 25.35 sent to C&DA. However Kharif 2019 34.85 Crores Crores 73% Amount credited to 23951 accounts by C&DA to the extent of budget released. 21307 Far 18327 86 % Rabi 2019- 20 Under progress at C&DA level 23.53 Crore 13.69Cr 56% 2 Rythu Bima 39640 39640 a -

Medchal District
MEDCHAL – MALKAJGIRI 2019 We acknowledge the content from medchal.telangana.gov.in Medchal−Malkajgiri is a district in the Indian state of Telangana. Medchal is the headquarter of the district[2]. It was a part of Ranga Reddy district before the re-organisation of districts in the state on 11 October 2016.[3] Medchal – Malkajgiri was carved out of erstwhile Ranga Reddy district. Medchal – Malkajgiri is a well connected district with 3 national highways and 2 state highways apart from many roadways running across the district. Medchal – Malkajgiri district, with 25 lakh population, is the second most populous and also 91.40% Urbanized district in the state of Telangana, standing next only to Hyderabad district ( 40 lakhs). The district is made up of 2 revenue divisions and 14 mandals. The district is spread over an area of 1,084 square kilometres (419 sq mi).It is situated 505 metres above sea level. The landmark consists of rocky terrain and monolithic rocks in the outskirts of the city As of 2011 census of India, the district is the second most populous district in the state with a population of 2,440,073] The district will have two revenue divisions of Keesara and Malkajgiri. They are sub- divided into 14 mandals.[3] M.V.Reddy is the present collector of the district. # Malkajgiri revenue division # Keesara revenue division 1 Alwal 1 Ghatkesar 2 Bachupally 2 Kapra 3 Balanagar 3 Keesara 4 Dundigal Gandimaisamma 4 Medchal 5 Kukatpally 5 Medipally 6 Malkajgiri 6 Shamirpet 7 Quthbullapur 7 Uppal Medchal-Malkajgiri District Parameters State Medchal s.no I KEY CHARACTERISTICS 1 Geographical Area (Sq. -

IPR in Respect of Group 'A' Officers of NFC for the Year 2019
Page 1 IPR in respect of Group 'A' Officers of NFC for the Year 2019 DATE OF ANNUAL INCOME SL EMP DESIGNATI ACQUIRED IN THE MODE OF NAME DESCRIPTION AND LOCATION OF PROPERTY AREA VALUE ACQUISITIO FROM THE NO NO ON NAME OF ACQUISITION N PROPERTY H.No. 2-171, St.No 3, AO- 1 2985 T PADMAVATHI Sri Padmapriya Nilayam, Vaninagar, 1354.5 Sft 1,05,000 T.Padmavathi 05-07-1991 Gift Nil II(ACCTS) Malkajgiri,Hyderabad HOUSE, H.NO. C2-317, NFC NAGAR, 283 BADER HUSSAIN, 2 3219 BADER HUSSAIN FMAN(C) 10,00,000 05-06-1992 Purchase 0 GHATKESAR, RANGA REDDY DISTRICT SQ.YARDS SELF HOUSE, H.NO. 21-4-898/5, GULAB SINGH BOWLI, 215 BADER HUSSAIN, 45,00,000 28-08-2015 Purchase 0 HYDERABAD, TENLANGANA SQ.YARDS SELF 24-143/28/2, VISHNUPURI, MALKAJGIRI, 3 3607 M N V VISWANATH SO(G) 293 Sq. Yards 5,00,000 M.N.V. VISWANATH 26-10-1988 Purchase HYDERABAD-47 FLAT NO. 404, RAJ RESIDENCY, VIDYANAGAR, 1280 SFT 25,30,000 M.N.V. VISWANATH 12-03-2012 Purchase HYDERABAD SURVEY 475/R ETC, BASWAPUR PULKAL 4.35 Acres 5,85,000 M.N.V. VISWANATH 05-06-2018 Purchase MANDAL, RANGA REDDY B2-563,NFC Nagar, Ghatakeswar, RR District 4 3787 J GURUNADH SO(G) 250 sq yards 1,50,000 J Gurunadh (Self) 17-05-1991 mortigage 12000 (House) Plat No.113, SR-94, Seetharam Nagar, RK puram 220 sq. yards 3,50,000 J.Gurunadh (Self) 01-02-1995 Purchase Self Occupied PO, Malkajgiri, Secunderabad(house) Sevey No.157, Ankushapur village, Ghatakeswar,RR 686 Sq yards 4,92,000 J.Gurunadh (Self) 05-03-2010 Purchase Nil District (Plot) Naidu Residency, F.No 201, Dayanand Nagar, Mortigaged to reliance 1860 Sq feet 27,00,000 J Gurunadh (self) 21-03-2011 48000 Malkajgiri, Hyderabad (Flat) home finance YALAVARTHI Plot No.15, bit-1, Vivekananda Nagar, Kukatpally, 5 3857 FMAN(C) 267 sq. -

PS NO-5 Keesara.Pdf
Serial Number Name of State No.of Name of No.of Part of in Part where elector is Assembly Assembly Assembly where Relation Sex enrolled in Constituency constituency Constituency Name of Part of Assembly elector is Serial Type Name of local (Male/Fe Assembly where elector where elector where elector is Constituency where elector is enrolled Number First Name of Elector Surname of Elector Name of Relation (Father/M Date of Birth authority of which EPIC Number male/Oth Constituency (if is enrolled (if is enrolled (if enrolled (if enrolled (if enrolled in any (if in Part other/Oth elector is a member er) enrolled in any enrolled in any enrolled in any enrolled in any assembly constituency) enrolled er) assembly assembly assembly assembly in any constituency) constituency) constituency) constituency) assembly constitue ncy) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 224 Sanjeeva Reddy Manda Buchi Reddy Father Male 10/09/1968 ZPTC GHATKESAR AP322190954339 Telangana 43 Medchal 361 ZPHS, Boduppal 224 Mandal Parished Upper 508 Bhagyamma Bingi Jangaiah Yadav Husband F 01/07/1975 MPTC CHENGICHERLA AP322190894346 Telangana 43 Medchal 269 508 Primary School, Chengicherla Mandal Parished Upper 253 Sridevi Padigam Yadagiri Father F 01/01/1986 MPTC KONDAPUR AP322190801427 Telangana 43 Medchal 279 253 Primary School, Kondapur Mandal Parished Upper 10 Srisailam Narri Mallaiah Father M 06/04/1975 MPTC YAMNAMPET AP322190900175 Telangana 43 Medchal 276 10 Primary School, Yamnampet Mandal Parished Upper 187 Narsingrao Bethala Pentaiah Father M 28/04/1983 MPTC GHATKESAR-I -

Nalanda High School, 30-658/2 Chandragiri Colony, Neredmet
VENU S.No. Name of the employee Design Office/STN CODE Nalanda High School, 30-658/2 Chandragiri Colony, 1 A V RAMESH BABU JE/II/Drg CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 2 AMBRISH PATEL JE/II/Drg CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 3 ANUJ KUMAR DWIEDI JE/II/Drg CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 4 ARVIND SONI JE II/DRG CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 5 B K SARMA OS CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 6 B S SHOBA RANI SSE/Drg CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 7 B SAINARAYANA RAO JE/II/Drg CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 8 BHIMANA HOSANANI JE/DRG CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 9 CH PADMAKAR Steno-II CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nalanda High School, 30-658/2 Chandragiri Colony, 10 D KRISHNA JE II/DRG CSTE/HQ 31008 Neredmet, Secunderabad - 500056. (A.P.) Contact No: 9849203710 Nava Jyothi High School, Patwari Nagar, Beside L.S.Function D V RAGHUNATH Hall, Behind Y.M.S. -

Telangana State Information Commission
TELANGANA STATE INFORMATION COMMISSION (Under Right to Information Act, 2005) Samachara Hakku Bhavan, D.No.5-4-399, ‘4’ Storied Commercial Complex, Housing Board Building, Mojam Jahi Market, Hyderabad – 500 001. Phone Nos: 040-24740665 (o); 040-24740592(f) Appeal No. 9397/CIC/2018 Dated: 05-11-2019 Appellant : Sri S. Anji Reddy, Hyderabad-500062. Respondents : 1. The Public Information Officer (U/RTI Act, 2005) / O/o The Deputy Commissioner, Greater Hyderabad Municipal Corporation, Circle No. 1, Kapra, Hyderabad. 2. The Public Information Officer (U/RTI Act, 2005) / O/o The Tahsildar, Kapra Mandal, Medchal District. The Appellate Authority (U/RTI Act, 2005) / O/o The Zonal Commissioner, Greater Hyderabad Municipal Corporation, East Zone, L.B. Nagar, Hyderabad. O R D E R Sri S. Anji Reddy, Hyderabad-500062 has filed 2nd appeal dated 18-06-2018 which was received by this Commission on 21-06-2018 for not getting the information sought by him from the PIO-1 / O/o The Deputy Commissioner, Greater Hyderabad Municipal Corporation, Circle No. 1, Kapra, Hyderabad, PIO-2 / O/o The Tahsildar, Kapra Mandal, Medchal District and 1st Appellate Authority / O/o The Zonal Commissioner, Greater Hyderabad Municipal Corporation, East Zone, L.B. Nagar, Hyderabad. The brief facts of the case as per the appeal and other records received along with it are that the appellant herein filed an application dated 22-03-2018 before the PIO under Sec.6(1) of the RTI Act, 2005, requesting to furnish the information on the following points mentioned in his application: TSIC The Public Information Officer has given a reply to the appellant vide Lr.No.54/TPS/RTI/Cir- 1/GHMC/2018, dated 25-04-2018 and dated 05-06-2018 GHMC, Kapra, Circle 1, Town Planning Section transferred of application under section 6(3) of RTI Act, transfer to The Tahsildar, Kapra Mandal, Medchal Dist with a request to furnish the information as sought by the applicant directly. -

Greater Hyderabad Municipal Corporation Wards Sl.No
Greater Hyderabad Municipal Corporation Wards Sl.No. No. & Name of the Ward 1 01- Kapra 2 2-Dr.A.S.Rao Nagar 3 3-Cherlapally 4 04-Meerpet H.B.Colony 5 5-Mallapur 6 6-Nacharam 7 7-Chilkanagar 8 8-Habsiguda 9 9-Ramanthapur 10 10-Uppal 11 11-Nagole 12 12-Mansoorabad 13 13-Hayathnagar 14 14-B.N.Reddy 15 15-Vanasthalipuram 16 16-Hasthinapuram 17 17- Champapet 18 18- Lingojiguda 19 19-Saroornagar 20 20-R.K.Puram 21 21-Kothapet 22 22- Chaitaynapuri 23 23-Gaddiannaram 24 24-Saidabad 25 25-Moosarambagh 26 26-Old Malakpet 27 27-Akberbagh 28 28-Azampura 29 29-Chawani 30 30-Dabeerpura 31 31-Rein Bazar 32 32 - Pathergatti 33 33-Moghalpura 34 34-Talabchanchalam 35 35-Gowlipura 36 36 Lalitha bagh 37 37 Kurmagida 38 38 I S Sadan 39 39 Santosh Nagar 40 40-Riyasathnagar 41 41-Kanchanbagh 42 42 - Barkas 43 43 Chandrayagutta 44 44 Uppuguda 45 45-Jangamet 46 46-Falaknuma 47 47-Nawab Saheb Kunta 48 48-Shahalibanda 49 49-Ghansi Bazar 50 50-Begum Bazar 51 51-Goshamahal 52 52-Puranapool 53 53-Doodh Bowli 54 54-Jahanuma 55 55-Ramnasthpura 56 56-Kishanbagh 57 57-Suleman Nagar 58 58-Shastripuram 59 59-Mailardevpally 60 60-Rajendra Nagar 61 61-Attapur 62 62-Ziaguda 63 63-Mangalhat 64 64 Dattatreya Nagar 65 65 Karwan 66 66 Langer House 67 67 Golconda 68 68 Tolichowki 69 69 Nanal Nagar 70 70 Mehdipatnam 71 71 Gudimalkapur 72 72-Asif Nagar 73 73-Vijaynagar Colony 74 74 - Ahmednagar 75 75 - Red Hills 76 76 - Mallepally 77 77 Jambagh 78 78 Gunfoundry 79 79 Himayatnagar 80 80-Kachiguda 81 81 Nallakunta 82 82 Golnaka 83 83 Amberpet 84 84 Baghamberpet 85 85 Adikmet 86 86 -

List of Returning Officers & Asst. Returning Officers for 150 Wards Of
List of Returning Officers & Asst. Returning Officers for 150 Wards of GHMC Name of the Name of the Returning Officer & Name of the Assistant Returning Officer & Sl. Circle No. Ward & No. Designation, Department. (Sarva Sri/ Smt/ Designation, Department (Sarva Sri/ Smt/ No. attached Kum) Kum) P.Venkat Ram Reddy, GHM, ZPHS CIRCLE 1 - Chinta Niranjan Rao, AD, CPO Wing, Keesara, Medchal- 1 1 - Kapra Dammaiguda, Kapra, Medchal Malkajgiri Kapra Malkajgiri District. 9866609090 District. 9391010142 CIRCLE 1 - 2 - A.S.Rao Jonnala Padmavathy, MPDO, MPP Keesara, D. Narshimha, GHM, ZPHS Balajinagar, Kapra, 2 Kapra Nagar Medchal-Malkajgiri District. 9849903334 Medchal Malkajgiri District. 7893291679 CIRCLE 1 - Kota Suvidha, MPDO, MPP Muduchinthalapally, A. Srinivas, GHM, ZPHS Bogaram, Keesara, 3 3-Cherlapally Kapra Medchal-Malkajgiri District. 9121245697 Medchal Malkajgiri District. 9177262558 CIRCLE 1 - 4-Meerpet HB Radhapadmaja, Sectorial Officer-III, Keesara, O/o G.Ravinder, GHM, ZPHS Cheeryal, Keesara, 4 Kapra Colony DEO, Medchal – Malkajgiri district. 9247773330 Medchal Malkajgiri District. 9704033864 A.Vidyasagar, GHM, ZPHS Hydernagar, CIRCLE 1 - Yella Aruna Reddy, MPDO, MPP Ghatkesar, 5 5-Mallapur Kukatpally, Medchal Malkajgiri District. Kapra Medchal-Malkajgiri District. 9849903335 9849579162 R.Rajpal Singh, GHM, ZPHS Moosapet (B), CIRCLE 1 - T. Jhansi Rani, DBCDO, District BC Development 6 6-Nacharam Kukatpally, Medchal Malkajgiri District. Kapra Office, Medchal-Malkajgiri District. 8978597373 8919023114 CIRCLE 2 - V.Madhavilatha, Agriculture Officer, Keesara, District V. Kishan, GHM, ZPHS RP Colony, Kukatpally, 7 7-Chiluka Nagar Uppal Agriculture Office, Medchal-Malkajgiri Dist. 7288894641 Medchal Malkajgiri District. 9441118823 Name of the Name of the Returning Officer & Name of the Assistant Returning Officer & Sl. Circle No.