Diversity and Life-Cycle Analysis of Pacific Ocean Zooplankton by Videomicroscopy and DNA Barcoding: Crustacea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Decapod Crustacean Grooming: Functional Morphology, Adaptive Value, and Phylogenetic Significance
Decapod crustacean grooming: Functional morphology, adaptive value, and phylogenetic significance N RAYMOND T.BAUER Center for Crustacean Research, University of Southwestern Louisiana, USA ABSTRACT Grooming behavior is well developed in many decapod crustaceans. Antennular grooming by the third maxillipedes is found throughout the Decapoda. Gill cleaning mechanisms are qaite variable: chelipede brushes, setiferous epipods, epipod-setobranch systems. However, microstructure of gill cleaning setae, which are equipped with digitate scale setules, is quite conservative. General body grooming, performed by serrate setal brushes on chelipedes and/or posterior pereiopods, is best developed in decapods at a natant grade of body morphology. Brachyuran crabs exhibit less body grooming and virtually no specialized body grooming structures. It is hypothesized that the fouling pressures for body grooming are more severe in natant than in replant decapods. Epizoic fouling, particularly microbial fouling, and sediment fouling have been shown r I m ans of amputation experiments to produce severe effects on olfactory hairs, gills, and i.icubated embryos within short lime periods. Grooming has been strongly suggested as an important factor in the coevolution of a rhizocephalan parasite and its anomuran host. The behavioral organization of grooming is poorly studied; the nature of stimuli promoting grooming is not understood. Grooming characters may contribute to an understanding of certain aspects of decapod phylogeny. The occurrence of specialized antennal grooming brushes in the Stenopodidea, Caridea, and Dendrobranchiata is probably not due to convergence; alternative hypotheses are proposed to explain the distribution of this grooming character. Gill cleaning and general body grooming characters support a thalassinidean origin of the Anomura; the hypothesis of brachyuran monophyly is supported by the conservative and unique gill-cleaning method of the group. -

From Ghost and Mud Shrimp
Zootaxa 4365 (3): 251–301 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4365.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C5AC71E8-2F60-448E-B50D-22B61AC11E6A Parasites (Isopoda: Epicaridea and Nematoda) from ghost and mud shrimp (Decapoda: Axiidea and Gebiidea) with descriptions of a new genus and a new species of bopyrid isopod and clarification of Pseudione Kossmann, 1881 CHRISTOPHER B. BOYKO1,4, JASON D. WILLIAMS2 & JEFFREY D. SHIELDS3 1Division of Invertebrate Zoology, American Museum of Natural History, Central Park West @ 79th St., New York, New York 10024, U.S.A. E-mail: [email protected] 2Department of Biology, Hofstra University, Hempstead, New York 11549, U.S.A. E-mail: [email protected] 3Department of Aquatic Health Sciences, Virginia Institute of Marine Science, College of William & Mary, P.O. Box 1346, Gloucester Point, Virginia 23062, U.S.A. E-mail: [email protected] 4Corresponding author Table of contents Abstract . 252 Introduction . 252 Methods and materials . 253 Taxonomy . 253 Isopoda Latreille, 1817 . 253 Bopyroidea Rafinesque, 1815 . 253 Ionidae H. Milne Edwards, 1840. 253 Ione Latreille, 1818 . 253 Ione cornuta Bate, 1864 . 254 Ione thompsoni Richardson, 1904. 255 Ione thoracica (Montagu, 1808) . 256 Bopyridae Rafinesque, 1815 . 260 Pseudioninae Codreanu, 1967 . 260 Acrobelione Bourdon, 1981. 260 Acrobelione halimedae n. sp. 260 Key to females of species of Acrobelione Bourdon, 1981 . 262 Gyge Cornalia & Panceri, 1861. 262 Gyge branchialis Cornalia & Panceri, 1861 . 262 Gyge ovalis (Shiino, 1939) . 264 Ionella Bonnier, 1900 . -

Estuarine Mudcrab (Rhithropanopeus Harrisii) Ecological Risk Screening Summary
Estuarine Mudcrab (Rhithropanopeus harrisii) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, February 2011 Revised, May 2018 Web Version, 6/13/2018 Photo: C. Seltzer. Licensed under CC BY-NC 4.0. Available: https://www.inaturalist.org/photos/4047991. (May 2018). 1 Native Range and Status in the United States Native Range From Perry (2018): “Original range presumed to be in fresh to estuarine waters from the southwestern Gulf of St. Lawrence, Canada, through the Gulf of Mexico to Vera Cruz, Mexico (Williams 1984).” 1 Status in the United States From Perry (2018): “The Harris mud crab was introduced to California in 1937 and is now abundant in the brackish waters of San Francisco Bay and freshwaters of the Central Valley (Aquatic Invaders, Elkhorn Slough Foundation). Ricketts and Calvin (1952) noted its occurrence in Coos Bay, Oregon in 1950. Rhithropanopeus harrisii, a common resident of Texas estuaries, has recently expanded its range to freshwater reservoirs in that state (Howells 2001; […]). They have been found in the E.V. Spence, Colorado City, Tradinghouse Creek, Possum Kingdom, and Lake Balmorhea reservoirs. These occurrences are the first records of this species in freshwater inland lakes.” From Fofonoff et al. (2018): “[…] R. harrisii has invaded many estuaries in different parts of the world, and has even colonized some freshwater reservoirs in Texas and Oklahoma, where high mineral content of the water may promote survival and permit reproduction (Keith 2006; Boyle 2010).” This species is in trade in the United States. From eBay (2018): “3 Freshwater Dwarf Mud Crabs Free Shipping!!” “Price: US $26.00” “You are bidding on 3 unsexed Freshwater Dwarf Mud Crabs (Rhithropanopeus harrisii).” Means of Introductions in the United States From Fofonoff et al. -

Diversity and Life-Cycle Analysis of Pacific Ocean Zooplankton by Video Microscopy and DNA Barcoding: Crustacea
Journal of Aquaculture & Marine Biology Research Article Open Access Diversity and life-cycle analysis of Pacific Ocean zooplankton by video microscopy and DNA barcoding: Crustacea Abstract Volume 10 Issue 3 - 2021 Determining the DNA sequencing of a small element in the mitochondrial DNA (DNA Peter Bryant,1 Timothy Arehart2 barcoding) makes it possible to easily identify individuals of different larval stages of 1Department of Developmental and Cell Biology, University of marine crustaceans without the need for laboratory rearing. It can also be used to construct California, USA taxonomic trees, although it is not yet clear to what extent this barcode-based taxonomy 2Crystal Cove Conservancy, Newport Coast, CA, USA reflects more traditional morphological or molecular taxonomy. Collections of zooplankton were made using conventional plankton nets in Newport Bay and the Pacific Ocean near Correspondence: Peter Bryant, Department of Newport Beach, California (Lat. 33.628342, Long. -117.927933) between May 2013 and Developmental and Cell Biology, University of California, USA, January 2020, and individual crustacean specimens were documented by video microscopy. Email Adult crustaceans were collected from solid substrates in the same areas. Specimens were preserved in ethanol and sent to the Canadian Centre for DNA Barcoding at the Received: June 03, 2021 | Published: July 26, 2021 University of Guelph, Ontario, Canada for sequencing of the COI DNA barcode. From 1042 specimens, 544 COI sequences were obtained falling into 199 Barcode Identification Numbers (BINs), of which 76 correspond to recognized species. For 15 species of decapods (Loxorhynchus grandis, Pelia tumida, Pugettia dalli, Metacarcinus anthonyi, Metacarcinus gracilis, Pachygrapsus crassipes, Pleuroncodes planipes, Lophopanopeus sp., Pinnixa franciscana, Pinnixa tubicola, Pagurus longicarpus, Petrolisthes cabrilloi, Portunus xantusii, Hemigrapsus oregonensis, Heptacarpus brevirostris), DNA barcoding allowed the matching of different life-cycle stages (zoea, megalops, adult). -

Biodiversity of Jamaican Mangrove Areas
BIODIVERSITY OF JAMAICAN MANGROVE AREAS Volume 7 Mangrove Biotypes VI: Common Fauna BY MONA WEBBER (PH.D) PROJECT FUNDED BY: 2 MANGROVE BIOTYPE VI: COMMON FAUNA CNIDARIA, ANNELIDA, CRUSTACEANS, MOLLUSCS & ECHINODERMS CONTENTS Page Introduction…………………………………………………………………… 4 List of fauna by habitat……………………………………………………….. 4 Cnidaria Aiptasia tagetes………………………………………………………… 6 Cassiopeia xamachana………………………………………………… 8 Annelida Sabellastarte magnifica………………………………………………… 10 Sabella sp………………………………………………………………. 11 Crustacea Calinectes exasperatus…………………………………………………. 12 Calinectes sapidus……………………………………………………… 13 Portunis sp……………………………………………………………… 13 Lupella forcepes………………………………………………………… 14 Persephone sp. …………………………………………………………. 15 Uca spp………………………………………………………………..... 16 Aratus pisoni……………………………………………………………. 17 Penaeus duorarum……………………………………………………… 18 Panulirus argus………………………………………………………… 19 Alphaeus sp…………………………………………………………….. 20 Mantis shrimp………………………………………………………….. 21 Balanus eberneus………………………………………………………. 22 Balanus amphitrite…………………………………………………….. 23 Chthamalus sp………………………………………………………….. 23 Mollusca Brachidontes exustus…………………………………………………… 24 Isognomon alatus………………………………………………………. 25 Crassostrea rhizophorae……………………………………………….. 26 Pinctada radiata………………………………………………………... 26 Plicatula gibosa………………………………………………………… 27 Martesia striata…………………………………………………………. 27 Perna viridis……………………………………………………………. 28 Trachycardium muricatum……………………………………..……… 30 Anadara chemnitzi………………………………………………...……. 30 Diplodonta punctata………………………………………………..…... 32 Dosinia sp…………………………………………………………..…. -

Lake Worth Lagoon
TABLE OF CONTENTS INTRODUCTION ................................................................................ 1 PURPOSE AND SIGNIFICANCE OF THE PARK ...................................... 1 Park Significance .............................................................................. 1 PURPOSE AND SCOPE OF THE PLAN ................................................... 2 MANAGEMENT PROGRAM OVERVIEW ................................................. 7 Management Authority and Responsibility ............................................ 7 Park Management Goals .................................................................... 8 Management Coordination ................................................................. 8 Public Participation ........................................................................... 9 Other Designations ........................................................................... 9 RESOURCE MANAGEMENT COMPONENT INTRODUCTION .............................................................................. 15 RESOURCE DESCRIPTION AND ASSESSMENT ................................... 15 Natural Resources .......................................................................... 16 Topography ............................................................................... 20 Geology .................................................................................... 21 Soils ......................................................................................... 21 Minerals ................................................................................... -

Upogebia Pugettensis Class: Malacostraca Order: Decapoda Section: Anomura, Paguroidea the Blue Mud Shrimp Family: Upogebiidae
Phylum: Arthropoda, Crustacea Upogebia pugettensis Class: Malacostraca Order: Decapoda Section: Anomura, Paguroidea The blue mud shrimp Family: Upogebiidae Taxonomy: Dana described Gebia (on either side of the mouth), two pairs of pugettensis in 1852 and this species was later maxillae and three pairs of maxillipeds. The redescribed as Upogebia pugettensis maxillae and maxillipeds attach posterior to (Stevens 1928; Williams 1986). the mouth and extend to cover the mandibles (Ruppert et al. 2004). Description Carapace: Bears two rows of 11–12 Size: The type specimen was 50.8 mm in teeth laterally (Fig. 1) in addition to a small length and the illustrated specimen (ovigerous distal spines (13 distal spines, 20 lateral teeth female from Coos Bay, Fig. 1) was 90 mm in on carapace shoulder, see Wicksten 2011). length. Individuals are often larger and reach Carapace with thalassinidean line extending sizes to 100 mm (range 75–112 mm) and from anterior to posterior margin (Wicksten northern specimens are larger than those in 2011). southern California (MacGinitie and Rostrum: Large, tridentate, obtuse, MacGinitie 1949; Wicksten 2011). rough and hairy (Schmitt 1921), the sides Color: Light blue green to deep olive brown bear 3–5 short conical teeth (Wicksten 2011). with brown fringes on pleopods and pleon. Rostral tip shorter than antennular peduncle. Individual color variable and may depend on Two short processes extending on either side feeding habits (see Fig. 321, Kozloff 1993; each with 0–2 dorsal teeth (Wicksten 2011). Wicksten 2011). Teeth: General Morphology: The body of decapod Pereopods: Two to five simple crustaceans can be divided into the walking legs. -
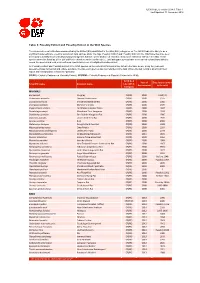
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2014.3: Table 9 Last Updated: 13 November 2014 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red Year of Date last recorded Scientific name Common name List (2014) Assessment in the wild Category MAMMALS Bos sauveli Kouprey CR(PE) 2008 1969/70 Crateromys australis Dinagat Crateromys CR(PE) 2008 1975 Crocidura trichura Christmas Island Shrew CR(PE) 2008 1985 Crocidura wimmeri -

Investigation of Maryland's Coastal Bays and Atlantic Ocean Finfish
Investigation of Maryland’s Coastal Bays and Atlantic Ocean Finfish Stocks July 2015 - June 2016 Final Report Prepared by: Steve Doctor, Gary Tyler, Craig Weedon, and Angel Willey Federal Aid Project No. F-50-R-24 UNITED STATES DEPARTMENT OF INTERIOR Fish & Wildlife Service Division of Federal Assistance Region 5 Final Performance Progress Report Investigation of Maryland’s Coastal Bays and Atlantic Ocean Finfish Stocks April 1, 2015 through June 30, 2016 Grantee: Maryland Department of Natural Resources – Fisheries Service Grant No.: F15AF00156 Segment No.: F-50-R-24 Title: Investigation of Maryland’s Coastal Bays and Atlantic Ocean Finfish Stocks Period Covered: July 1, 2015 through June 30, 2016 ______ Prepared By: Angel Willey, Principal Investigator & Manager Coastal Program Approved By: Michael Luisi, Director, Estuarine & Marine Division Approved By: David Blazer, Appointing Authority Date Submitted: Statutory Funding Authority: Sport Fish Restoration X CFDA #15.605 State Wildlife Grants (SWG) Cooperative Management Act CFDA #15.634 Acknowledgements The Coastal Bays Fisheries Investigation has been sampling fishes in the Coastal Bays for 43 years. Although the survey began in 1972, it did not have dedicated funding until 1989. Consistent funding allowed staff to specifically dedicate time and make improvements to the sampling protocol that resulted in significant beneficial contributions to the fisheries of the Coastal Bays. We would like to thank the past and present staff that dedicated their careers to the Coastal Bays Fisheries Investigation for having the knowledge, initiative, and dedication to get it started and maintained. Additionally, staff of the Coastal Fisheries Program would like to thank all of the Maryland Department of Natural Resources (MDNR) employees who assisted with the operations, field work, and annual reports over the years whether it was for a day or a few months. -

The Barnacle Amphibalanus Improvisus (Darwin, 1854), and the Mitten Crab Eriocheir: One Invasive Species Getting Off on Another!
BioInvasions Records (2015) Volume 4, Issue 3: 205–209 Open Access doi: http://dx.doi.org/10.3391/bir.2015.4.3.09 © 2015 The Author(s). Journal compilation © 2015 REABIC Rapid Communication The barnacle Amphibalanus improvisus (Darwin, 1854), and the mitten crab Eriocheir: one invasive species getting off on another! Murtada D. Naser1,4, Philip S. Rainbow2, Paul F. Clark2*, Amaal Gh. Yasser1,4 and Diana S. Jones3 1Marine Biology Department, Marine Science Centre, University of Basrah, Basrah, Iraq 2Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, England 3Western Australian Museum, 49 Kew Street, Welshpool, Western Australia, 6106 Australia 4Australian Rivers Institute, Griffith University, 170 Kessels Road, Nathan, Queensland, 4111 Australia E-mail: [email protected] (MDN), [email protected] (PSR), [email protected] (PFC), [email protected] (AGY), [email protected] (DSJ) *Corresponding author Received: 9 March 2015 / Accepted: 20 May 2015 / Published online: 16 June 2015 Handling editor: Vadim Panov Abstract The balanoid barnacle, Amphibalanus improvisus (Darwin, 1854), was found on the carapaces of two invasive species of mitten crabs: Eriocheir sinensis H. Milne Edwards, 1853 and E. hepuensis Dai, 1991. The first instance was from a female mitten crab captured from the River Thames estuary, Kent, England, where A. improvisus is common. However, the second record, on a Hepu mitten crab from Iraq is the first record of A. improvisus from the Persian Gulf. Key words: Eriocheir sinensis H. Milne Edwards, 1853, E. hepuensis Dai, 1991, invasive species, England, Iraq, barnacles, mitten crabs Introduction the eastern and western North Atlantic; Baltic Sea; west coast of Africa (to the Cape of Good “Hairy” (Southeast and East Asia) or “mitten” Hope); Mediterranean Sea; Black Sea; Caspian (Europe) crabs are currently assigned to Eriocheir Sea; Red Sea; Straits of Malacca; Singapore; De Haan, 1835 (Brachyura: Grapsoidea: Varunidae). -
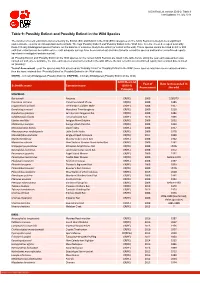
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2019-2: Table 9 Last Updated: 18 July 2019 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". Year of Assessment - year the species was first assessed as 'Possibly Extinct' or 'Possibly Extinct in the Wild'; some species may have been reassessed since then but have retained their 'Possibly Extinct' or 'Possibly Extinct in the Wild' status. CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red List Year of Date last recorded in