On-Site COVID-19 Testing with the ID NOW
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

British Columbia Alberta
94J3 94J2 94J1 94I494I 94I3 94I2 94I1 94G14 94G15 94G16 94H13 94H14 94H15 94H16 94G11 94G10 94G9 94H12 94H11 94H10 94H9 97 94G6 94G7 94G8 96 94H5 94H6 96 94H7 94H8 95 95 94 94G6 94 LATERAL COMPRESSOR RECEIPT POINT LEGEND 94G2 94G1 RECEIPT RECEIPT PT RECEIPT POINT METER PLANT 94H494H STATION LOCATIONS 93 1A 2A 94H3 POINT MNEMONIC NAME - OPERATOR LOCATION LATERAL NAME LOCATION 94H2 93 94H1 1 2 BC 01 HIWAY HIGHWAY - WGSI d-37-I 94-B-16 HIGHWAY d-36-I 94-B-16 W No. NAME a 92 b BC 01A HWAY2 HIGHWAYas 2 - WGSI d-37-I 94-B-16 HIGHWAY d-36-I 94-B-16 92 ca BC 02 ATKCK AITKEN CREEK - PIONEER d-44-L 94-A-13 AITKEN CREEK d-44-L 94-A-13 94B14 91 AB21 TEEPEE CREEK BC 02A AKCK2 AITKEN CREEK - UNOCALRiver d-44-L 94-A-13 AITKEN CREEK d-44-L 94-A-13 94B15 91 BC 03 MCMAN McMAHON - DUKE 16-25-82-18 W6 McMAHON 16-25-82-18 W6 94B16 WONOWON AB30 GOLD CREEK 94A13 BUICK BC 04 YUNGR YOUNGER - TAYLOR 1-36-82-18 W6 TAYLOR 1-36-82-18 W6 94A14 94 A 90 94A15 BC 12 WESDO WEST DOE - SPECTRA (Q4 '07) 12-24-80-15 W6 FT. ST. JOHN 2-25-80-15 W6 94A16 90 AB47 CARSON CREEK AB 05 BDLYK BOUNDARY - PETROCAN 11-24-84-15 W6 BOUNDARY LAKE 14-24-84-15 W6 BLUEBERRY AB48 WHITECOURT 89 RIVER AB 06 BDLK2 BOUNDARY LK. - I.O.L. -

Minutes of the Special Meeting of the Council
MINUTES OF COMMITTEE OF THE WHOLE MEETING COUNCIL CHAMBERS Tuesday, May 14, 2019 at 1:00 p.m. _______________________________________________________________________________________ Council Present: Mayor Tyler Warman and Councillors: Brice Ferguson Rebecca King, Shawn Gramlich, Darin Busk, Joy McGregor, and Julie Brandle Staff Present: Roland Schmidt – Acting CAO, Doug Baird – Projects Manager, Christopher Brown - Communications Coordinator, Garry Roth - Director of Community Services, Jill Hutchings - Community Relations Manager, Kirsten Coutts - Administrative Services Coordinator, Laurie Skrynyk - Director of Planning and Development, Vanessa Asselin - Planning and Development Officer, and Briana Lachance – Recording Secretary. Media Present: Lakeside Leader Others Present: Residents Meeting called to order at 1:01 p.m. by. Addition: Coffee with Council Agenda: Motion #173-19: Moved by Councillor King That the Committee of the Whole Agenda for May 14, 2019 be accepted as amended. CARRIED Introductions CAO Update: May 14, 2019 Safety: • Health and safety met with NLAC staff on May 13th. Topics discussed included proper incident reporting procedures, and emergency response for various scenarios. • The Workplace Harassment and Discrimination policy draft is complete and is currently being reviewed before presenting it to the M10. • Following up on the completion of corrective actions arising from formal workplace inspections and incident reports. Administration: • The Engineer for our DRP claims was here on Monday to visit all of the sites. • The Alberta Environment people for the flood mitigation plan were here last week to tour the creek berms and trash racks. Surveying will start in a few weeks. Finance: • We have been getting lots of questions on the taxes and especially on the Utility bills. -

Swan Hills - Fox Creek
Alberta Early Development Instrument Community Profile Report 2016 Data Collection Whitecourt - Swan Hills - Fox Creek Community wide data not available. Only Sub-community C participated in the 2016 EDI Collection, therefore no sub-community reports are provided and the Community Report only represents sub-community C. Alberta Health February 2018 EDI Community Profile: WHITECOURT - SWAN HILLS - FOX CREEK Acknowledgement We wish to extend our greatest appreciation to all of our partners for their hard work and commitment to the Alberta Early Development Instrument (EDI) Program. A very special thank you to the Early Childhood Coalitions of Alberta as well as Family and Community Support Services Association of Alberta (FCSSAA) and their support staff for everything they do to support dissemination of these reports throughout Alberta's local communities. To all of the incredible teachers who have committed their time and energy to filling out EDI questionnaires, we express our sincere gratitude. Without you, none of this would be possible. The Community Profiles use currently available 2016 EDI data. For more information, please contact Alberta Connects https://informalberta.ca/public/service/serviceProfileStyled.do?serviceQueryId=1049614 Contributors (Alphabetic by Family Name, within Branch/Organization): Jennifer Bian - Analytics and Performance Reporting; Alberta Health Gary Gilham - Analytics and Performance Reporting; Alberta Health Katherine Lyman - Analytics and Performance Reporting; Alberta Health Dan Metes - Analytics and Performance -
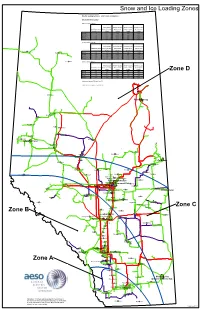
Wet Snow and Wind Loading
Snow and Ice Loading Zones Weather Loading Summary - AESO Tower Development Wet Snow & Wind Loadings 100 Year Return Values Wind Speed Wind Pressure Wind Pressure Wind Pressure Radial Wet Snow (km/hr) at 10m (Pa) at 20 m (Pa) at 30 m (Pa) at 40 m Accretion (mm) Height Height Height Height Zone A 70 77 295 320 340 Zone B 70 71 240 260 280 Zone C 50 67 210 230 245 Zone D 50 64 190 205 220 75 Year Return Values Wind Speed Wind Pressure Wind Pressure Wind Pressure Radial Wet Snow (km/hr) at 10m (Pa) at 20 m (Pa) at 30 m (Pa) at 40 m Accretion (mm) Height Height Height Height Rainbow Lake High Level Zone A 65 75 270 290 310 Zone B 65 70 235 255 270 Zone C 45 65 200 215 230 Zone D 45 62 180 195 210 La Crète 50 Year Return Values Wind Speed Wind Pressure Wind Pressure Wind Pressure Radial Wet Snow (km/hr) at 10m (Pa) at 10 m (Pa) at 20 m (Pa) at 30 m Accretion (mm) Height Height Height Height Zone A 60 74 220 255 280 Zone D Zone B 60 69 190 220 240 Zone C 40 63 160 185 200 Zone D 40 60 145 170 185 Wet snow density 350 kg/m3 at -5C Table Data Last Update: 2010-03-25 Manning Fort McMurray Peace River Grimshaw Fairview Spirit River Falher McLennan High Prairie Sexsmith Beaverlodge Slave Lake Grande Prairie Valleyview Lac la Biche Swan Hills Athabasca Cold Lake Fox Creek Bonnyville Westlock Whitecourt Barrhead Smoky Lake St. -

CP's North American Rail
2020_CP_NetworkMap_Large_Front_1.6_Final_LowRes.pdf 1 6/5/2020 8:24:47 AM 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Lake CP Railway Mileage Between Cities Rail Industry Index Legend Athabasca AGR Alabama & Gulf Coast Railway ETR Essex Terminal Railway MNRR Minnesota Commercial Railway TCWR Twin Cities & Western Railroad CP Average scale y y y a AMTK Amtrak EXO EXO MRL Montana Rail Link Inc TPLC Toronto Port Lands Company t t y i i er e C on C r v APD Albany Port Railroad FEC Florida East Coast Railway NBR Northern & Bergen Railroad TPW Toledo, Peoria & Western Railway t oon y o ork éal t y t r 0 100 200 300 km r er Y a n t APM Montreal Port Authority FLR Fife Lake Railway NBSR New Brunswick Southern Railway TRR Torch River Rail CP trackage, haulage and commercial rights oit ago r k tland c ding on xico w r r r uébec innipeg Fort Nelson é APNC Appanoose County Community Railroad FMR Forty Mile Railroad NCR Nipissing Central Railway UP Union Pacic e ansas hi alga ancou egina as o dmon hunder B o o Q Det E F K M Minneapolis Mon Mont N Alba Buffalo C C P R Saint John S T T V W APR Alberta Prairie Railway Excursions GEXR Goderich-Exeter Railway NECR New England Central Railroad VAEX Vale Railway CP principal shortline connections Albany 689 2622 1092 792 2636 2702 1574 3518 1517 2965 234 147 3528 412 2150 691 2272 1373 552 3253 1792 BCR The British Columbia Railway Company GFR Grand Forks Railway NJT New Jersey Transit Rail Operations VIA Via Rail A BCRY Barrie-Collingwood Railway GJR Guelph Junction Railway NLR Northern Light Rail VTR -

Mayerthorpe & Area Information Guide
MMaayyeerrtthhoorrppee && AArreeaa IInnffoorrmmaattiioonn GGuuiiddee Mayerthorpe will adapt and evolve to celebrate its history while growing its future. Table of Contents Message from the Mayor ........................................................................ pg.3 Introduction to Mayerthorpe ................................................................. pg.4 Government Services .............................................................................. pg.5-6 Taxes ....................................................................................................... pg.6 Location .................................................................................................. pg.6 Climate .................................................................................................... pg.7 Population ............................................................................................... pg.7 Housing ................................................................................................... pg.7 Local Media ............................................................................................. pg.8 Medical .................................................................................................... pg.8 Education ................................................................................................ pg.9 Recreation Facilities ............................................................................... pg.9 Services and Utilities ............................................................................. -

2018 Municipal Affairs Population List | Cities 1
2018 Municipal Affairs Population List | Cities 1 Alberta Municipal Affairs, Government of Alberta November 2018 2018 Municipal Affairs Population List ISBN 978-1-4601-4254-7 ISSN 2368-7320 Data for this publication are from the 2016 federal census of Canada, or from the 2018 municipal census conducted by municipalities. For more detailed data on the census conducted by Alberta municipalities, please contact the municipalities directly. © Government of Alberta 2018 The publication is released under the Open Government Licence. This publication and previous editions of the Municipal Affairs Population List are available in pdf and excel version at http://www.municipalaffairs.alberta.ca/municipal-population-list and https://open.alberta.ca/publications/2368-7320. Strategic Policy and Planning Branch Alberta Municipal Affairs 17th Floor, Commerce Place 10155 - 102 Street Edmonton, Alberta T5J 4L4 Phone: (780) 427-2225 Fax: (780) 420-1016 E-mail: [email protected] Fax: 780-420-1016 Toll-free in Alberta, first dial 310-0000. Table of Contents Introduction ..................................................................................................................................... 4 2018 Municipal Census Participation List .................................................................................... 5 Municipal Population Summary ................................................................................................... 5 2018 Municipal Affairs Population List ....................................................................................... -

Fish Stocking Report, 2020 (Final)
Fish Stocking Report 2020 (Final) Fish stocking managed by the Government of Alberta and the Alberta Conservation Association Updated February 18, 2021 Notes There are no cutthroat trout stocked in the 2020 stocking season, as we will not be operating the Job Lake spawn camp due to COVID-19 restrictions. Average Length = adult fish stocked. Reference Species Stocked Strains Stocked Ploidy Stocked ARGR = Arctic Grayling BEBE = Beity x Beity TLTLJ = Trout Lodge / Jumpers 2N = diploid BKTR = Brook Trout BRBE = Bow River x Beity TLTLK = Trout Lodge / Kamloops 3N = triploid BNTR = Brown Trout CLCL = Campbell Lake TLTLS = Trout Lodge / Silvers AF2N = all female diploid CTTR = Cutthroat Trout JLJL = Job Lake AF3N = all female triploid RNTR = Rainbow Trout LYLY = Lyndon TGTR = Tiger Trout PLPL = Pit Lakes For further information on Fish Stocking visit: https://mywildalberta.ca/fishing/fish-stocking/default.aspx ©2021 Government of Alberta | Published: February 2021 Page 1 of 24 Waterbody Waterbody ATS Species Strain Genotype Average Number Stocking Official Name Common Name Length Stocked Date (2020) ALFORD LAKE SW4-36-8-W5 RNTR Campbell Lake 3N 18 3000 18-May-20 BEAR POND NW36-14-4-W5 RNTR Trout Lodge/Jumpers AF3N 19.7 750 22-Jun-20 BEAUVAIS LAKE SW29-5-1-W5 RNTR Trout Lodge/Jumpers AF3N 16.3 23000 11-May-20 BEAVER LAKE NE16-35-6-W5 RNTR Trout Lodge/Jumpers AF3N 21.3 2500 21-May-20 BEAVER LAKE NE16-35-6-W5 TGTR Beitty/Bow River 3N 16.9 500 02-Sep-20 BEAVER LAKE NE16-35-6-W5 TGTR Beitty/Bow River 3N 20 500 02-Sep-20 BEAVER MINES LAKE NE11-5-3-W5 -

Barrhead and Area Community Resources Proudly Sponsored by the Town of Barrhead Please Note: This List May Need to Be Updated
Recreation, Pool or Agrena Services: Barrhead and Area Community Resources proudly sponsored by The Town of Barrhead Please note: This list may need to be updated. If you have any changes or additions please contact the Barrhead Leader @ (780) 674-3823; [email protected] RCMP/FIRE/AMBULANCE (Emergency Only) ............................... 911 Barrhead & District FCSS ....................................................780-674-3341 Edmonton John Howard Society - Crisis Services Family Violence Prevention ................................... 1-780-423-1635 Alberta Mental Health Help Line ..................................... 1-877-303-2642 RCMP - Barrhead Detachment ..............780-674-2696 or 780-674-4848 Addiction Services - 24 Hour Help Line for Alcohol, Rural Crime Watch ...............780-674-4848 or [email protected] Drugs and Gambling ............................................. 1-866-332-2322 Treatment Group for Men who Batter (Whitecourt) ........ 1-780-778-6300 Barrhead Mental Health Services .......................................780-674-8243 Barrhead Healthcare Centre (HOSPITAL) .........................780-674-2221 HEALTH SERVICES Bullying Hotline ................................................................ 1-888-456-2323 Addiction Services (AADAC) ...............................................780-674-8239 Child Abuse AIDS/Sexually Transmitted Disease ............................... 1-800-772-2437 24 Hour Reporting Line .......................................... 1-800-387-5437 Barrhead Clinic ....................................................................780-674-2231 -

2008 Wmus 505 and 507 Moose
2008 WMUs 505 and 507 moose Section Authors: Hugh Wollis and Curtis Stambaugh Suggested citation: Wollis, H. and C. Stambaugh. 2009. WMUs 505 and 507 moose. Pages 87‐91. In: N. Webb and R. Anderson. Delegated aerial ungulate survey program, 2007‐2008 survey season. Data Report, D‐2009‐008, produced by the Alberta Conservation Association, Rocky Mountain House, Alberta, Canada. 97 pp. Wildlife Management Unit 507 is a very popular hunting area for local residents and urban dwellers from nearby Edmonton. Previous surveys were conducted in 1995, 1999 and 2004. In 2006, a significant change in moose management occurred with the elimination of the General Moose Archery season. This change caused concerns from local archery hunters who met with the Minister, citing among other things poor and out‐of‐date data for aerial surveys. The Minister supported the season change but agreed to give 507 a high priority for aerial surveys in 2008. Wildlife Management Unit 505, which is adjacent to WMU 507, had been flown in conjunction with 507 in 2004 and was also surveyed in 2008 in order to coordinate aircraft charters and reduce overall survey costs. Study area Wildlife Management Unit 507 extends roughly from Whitecourt to Barrhead, which is 100 km northwest of Edmonton (Figure 18). The WMU is bisected by the Athabasca River, an important feature for wildlife. The WMU is in the Boreal Mixedwood natural region and is a mixture of patented agricultural land east of the Athabasca where the land is primarily dominated by forage crops and pasture with some grain. West of the river the land is primarily Crown with some patented land that features mostly forage and pasture land near Ft. -

Long Term Adequacy Report
Long Term Adequacy Report August 2020 Introduction The following report provides information on the long term adequacy of the Alberta electric energy market. The report contains metrics that include tables on generation projects under development and generation retirements, an annual reserve margin with a five year forecast period, a two year daily supply cushion, and a two year probabilistic assessment of the Alberta Interconnected Electric System (AIES). The Long Term Adequacy (LTA) Metrics provide an assessment and information that can be used to facilitate further assessments of long term adequacy. This report is updated quarterly in February, May, August, and November. Inquiries on the report can be made at [email protected]. *All metrics have been updated and use the most recent corporate load forecast the 2019 LTO found at https://www.aeso.ca/grid/forecasting. Summary of Changes since Previous Report New Generation and Retirements Metric Projects completed and removed from list: Keyera West Pembina 359S Gas Turbine Gas FortisAlberta 895S Suffield DG PV FortisAlberta Innisfail 214S DER Solar Maxim Power Milner Gas FortisAlberta 257S Hull DER Solar FortisAlberta Vauxhall Solar DER ENMAX Shepard Gas Upgrade Generation Projects moved to “Active Construction”: ENMAX Crossfield CRS3 Battery Storage Greengate Travers MPC Solar TransAlta Windrise MPC Wind Generation projects moved to “Regulatory Approval”: TransAlta Sundance Unit 5 Gas TransAlta Keephills Unit 1 Gas FortisAlberta Fieldgate 824S DER Gas Sequoia Energy Schuler -

Southern Alberta
R.3 R.1W.4M. R.7 R.5 R.11 R.9 R.17 R.15 R.13 OYEN T.27 R.23 R.21 R.19 R.9 R.7 R.5 R.3 R.1W.5M. R.27 R.25 LANFINE NORTH 2 21 WINTERING HILLS BULLPOUND BANFF T.25 WHEATLAND 9 56 LANFINE SOUTH PARK 1 EAST 22 STRATHMORE 36 CALGARY T.23 STRATHMORE1 SUNSHINE COLONY AUC FARMING CO. LTD. Alberta Utilities Commission BARLOW GLEICHEN WHEATCREST T.21 JENNER JENNER T.21 LATHOM BUFFALO ATLEE EMPRESS SOUTHERN ALBERTA WIND FARMS SUNALTA BUFFALO ATLEE 4 41 T.19 1 SADDLEBROOK BROOKS SOLAR II T.19 BROOKS SOLAR I HILDA AND SOLAR PROJECTS 22 TILLEY HIGH RIVER BROOKS PRAIRIE SUNLIGHT BROOKS T.17 FIFTH MERIDIAN SCHULER FOURTH MERIDIAN SEPTEMBER 2021 T.17 2 VULCAN VULCAN BUFFALO PLAINS 1 ENTERPRISE 36 T.15 KIRKCALDY PRAIRIE SUNLIGHT I SUFFIELD WIND FARM IN-SERVICE CHAPPICE T.15 TRAVERS R.3 R.7 R.5 R.11 R.9 WIND FARM APPROVED R.15 R.13 DUNMORE STAVELY R.25 R.23 R.19 R.17 T.13 R.5 R.3 R.29 VAUXHALL BOX SPRINGS WIND FARM APPLIED FOR BLACKSPRING HAYS MEDICINE HAT T.13 CLARESHOLM RIDGE PRAIRIE SUNLIGHT III VULCAN WINNIFRED 1 SOLAR PROJECT IN-SERVICE CLARESHOLM PRAIRIE SUNLIGHT II T.11 CYPRESS FORTY MILE WILD ROSE 1 SOLAR PROJECT APPROVED RATTLESNAKE3 T.11 2 BOW ISLAND RIDGE 22 ALBERTA SOLAR ONE BURDETT SOLAR PROJECT APPLIED FOR TABER T.9 MONARCH COALDALE SUNSET 41 3 PEACE BUTTE 3 WEATHER DANCER 1 FORT MACLEOD TABER WHITLA WILD ROSE 2 SUMMERVIEWT.9 MACLEOD FLATS CHIN CHUTE YELLOW T.7 BLUE TRAIL LAKE SUNCOR FORTY MILE LETHBRIDGE STIRLING COWLEY RIDGE RIVERVIEW McBRIDE LAKE LUNDBRECK ARDENVILLE 3 36 PRAIRIE HOME CASTLE ROCK RIDGE WRENTHAM Scale 1:250 000 SINNOTT SODERGLEN 4 T.5 CASTLE RIVER PINCHER WINDRISE CREEK KETTLES HILL MAGRATH OPTIMIST km 10 0 10 20 30 40 50 60 70 MCLAUGHLIN2 OLDMAN OLDMAN 2 OK HUTTERIAN miles 10 0 10 20 30 40 50 OLD ELM BRETHREN COLONY RIVER WARNER 41 T.3 BLUE RIDGE WEST BELLY RIVER BEND BLUE RIDGE SPRING COULEE PRODUCED BY AUC MAPPING SECTION T.3 WATERTON BELLY RIVER CARDSTON WATERTON SOUTH T.1.