Why Cachexia Kills: Examining the Causality of Poor Outcomes in Wasting Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scurvy Due to Selective Diet in a Seemingly Healthy 4-Year-Old Boy Andrew Nastro, MD,A,G,H Natalie Rosenwasser, MD,A,B Steven P
Scurvy Due to Selective Diet in a Seemingly Healthy 4-Year-Old Boy Andrew Nastro, MD,a,g,h Natalie Rosenwasser, MD,a,b Steven P. Daniels, MD,c Jessie Magnani, MD,a,d Yoshimi Endo, MD,e Elisa Hampton, MD,a Nancy Pan, MD,a,b Arzu Kovanlikaya, MDf Scurvy is a rare disease in developed nations. In the field of pediatrics, it abstract primarily is seen in children with developmental and behavioral issues, fDivision of Pediatric Radiology and aDepartments of malabsorptive processes, or diseases involving dysphagia. We present the Pediatrics and cRadiology, NewYork-Presbyterian/Weill Cornell Medical Center, New York, New York; bDivision of case of an otherwise developmentally appropriate 4-year-old boy who Pediatric Rheumatology and eDepartment of Radiology, developed scurvy after gradual self-restriction of his diet. He initially Hospital for Special Surgery, New York, New York; and d presented with a limp and a rash and was subsequently found to have anemia Division of Neonatal-Perinatal Medicine, University of Michigan, Ann Arbor, Michigan gDepartment of Pediatrics, and hematuria. A serum vitamin C level was undetectable, and after review of NYU School of Medicine, New York, New York hDepartment of the MRI of his lower extremities, the clinical findings supported a diagnosis of Pediatrics, Bellevue Hospital Center, New York, New York scurvy. Although scurvy is rare in developed nations, this diagnosis should be Dr Nastro helped conceptualize the case report, considered in a patient with the clinical constellation of lower-extremity pain contributed to writing the introduction, initial presentation, hospital course, and discussion, and or arthralgias, a nonblanching rash, easy bleeding or bruising, fatigue, and developed the laboratory tables while he was anemia. -

Iron Deficiency and the Anemia of Chronic Disease
Thomas G. DeLoughery, MD MACP FAWM Professor of Medicine, Pathology, and Pediatrics Oregon Health Sciences University Portland, Oregon [email protected] IRON DEFICIENCY AND THE ANEMIA OF CHRONIC DISEASE SIGNIFICANCE Lack of iron and the anemia of chronic disease are the most common causes of anemia in the world. The majority of pre-menopausal women will have some element of iron deficiency. The first clue to many GI cancers and other diseases is iron loss. Finally, iron deficiency is one of the most treatable medical disorders of the elderly. IRON METABOLISM It is crucial to understand normal iron metabolism to understand iron deficiency and the anemia of chronic disease. Iron in food is largely in ferric form (Fe+++ ) which is reduced by stomach acid to the ferrous form (Fe++). In the jejunum two receptors on the mucosal cells absorb iron. The one for heme-iron (heme iron receptor) is very avid for heme-bound iron (absorbs 30-40%). The other receptor - divalent metal transporter (DMT1) - takes up inorganic iron but is less efficient (1-10%). Iron is exported from the enterocyte via ferroportin and is then delivered to the transferrin receptor (TfR) and then to plasma transferrin. Transferrin is the main transport molecule for iron. Transferrin can deliver iron to the marrow for the use in RBC production or to the liver for storage in ferritin. Transferrin binds to the TfR on the cell and iron is delivered either for use in hemoglobin synthesis or storage. Iron that is contained in hemoglobin in senescent red cells is recycled by binding to ferritin in the macrophage and is transferred to transferrin for recycling. -

Iron Deficiency Anemia: Evaluation and Management MATTHEW W
Iron Deficiency Anemia: Evaluation and Management MATTHEW W. SHORT, LTC, MC, USA, and JASON E. DOMAGALSKI, MAJ, MC, USA Madigan Healthcare System, Tacoma, Washington Iron deficiency is the most common nutritional disorder worldwide and accounts for approxi- mately one-half of anemia cases. The diagnosis of iron deficiency anemia is confirmed by the findings of low iron stores and a hemoglobin level two standard deviations below normal. Women should be screened during pregnancy, and children screened at one year of age. Supple- mental iron may be given initially, followed by further workup if the patient is not responsive to therapy. Men and postmenopausal women should not be screened, but should be evaluated with gastrointestinal endoscopy if diagnosed with iron deficiency anemia. The underlying cause should be treated, and oral iron therapy can be initiated to replenish iron stores. Paren- teral therapy may be used in patients who cannot tolerate or absorb oral preparations. (Am Fam Physician. 2013;87(2):98-104. Copyright © 2013 American Academy of Family Physicians.) ▲ Patient information: ron deficiency anemia is diminished red causes of microcytosis include chronic A handout on iron defi- blood cell production due to low iron inflammatory states, lead poisoning, thalas- ciency anemia, written by 1 the authors of this article, stores in the body. It is the most com- semia, and sideroblastic anemia. is available at http://www. mon nutritional disorder worldwide The following diagnostic approach is rec- aafp.org/afp/2013/0115/ I and accounts for approximately one-half of ommended in patients with anemia and is p98-s1.html. Access to anemia cases.1,2 Iron deficiency anemia can outlined in Figure 1.2,6-11 A serum ferritin level the handout is free and unrestricted. -

The Relationship Between Serum Vitamin D Level, Anemia, and Iron Deficiency in Preschool Children
HAYDARPAŞA NUMUNE MEDICAL JOURNAL DOI: 10.14744/hnhj.2019.48278 Haydarpasa Numune Med J 2019;59(3):220–223 ORIGINAL ARTICLE hnhtipdergisi.com The Relationship Between Serum Vitamin D Level, Anemia, and Iron Deficiency in Preschool Children Ömer Kartal, Orhan Gürsel Department of Pediatric Hematology and Oncology, Gulhane Training and Research Hospital, Ankara, Turkey Abstract Introduction: Vitamin D deficiency and iron deficiency are the most common nutritional pandemic problems worldwide at all levels of society. In some studies, vitamin D has been shown to have an effect on erythropoiesis. The objective of this study was to investigate the relationship between serum vitamin D level, hemogram parameters, and serum iron level in preschool children. Methods: The study group comprised 108 children aged between 2 and 5 years who visited a single pediatric hematology polyclinic between August 2014 and August 2017and whose serum vitamin D level and iron parameters were evaluated. The patients were divided into 3 groups according to the hemoglobin value, serum ferritin level, and transferrin saturation index calculation: iron deficiency, iron deficiency anemia, and a control group. Vitamin D deficiency, insufficiency, and normal cate- gories were also used based on assessment of the serum vitamin D level. Results: There were 41 children in the iron deficiency group, 32 classified as iron deficiency anemia, and 35 age- and sex- mated controls. The vitamin D level was statistically significant between the groups (p<0.05). Discussion and Conclusion: According to our findings, vitamin D deficiency and insufficiency were prevalent, especially in children with iron deficiency anemia. It is recommended that the serum vitamin D level of children with iron deficiency ane- mia should be checked and vitamin D-fortified food consumption should be increased. -

WHO Technical Consultation on Folate and Vitamin B12 Deficiencies
Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies All participants in the Consultation Key words: Folate, vitamin B12 The consultation agreed on conclusions in four areas: » Indicators for assessing the prevalence of folate and Preamble vitamin B12 deficiencies » Health consequences of folate and vitamin B12 defi- Folate and vitamin B12 deficiencies occur primarily as ciencies a result of insufficient dietary intake or, especially in » Approaches to monitoring the effectiveness of inter- the case of vitamin B12 deficiency in the elderly, poor ventions absorption. Folate is present in high concentrations » Strategies to improve intakes of folate and vitamin B12 in legumes, leafy green vegetables, and some fruits, so lower intakes can be expected where the staple diet consists of unfortified wheat, maize, or rice, and when Indicators for assessing and monitoring the intake of legumes and folate-rich vegetables and vitamin status fruits is low. This situation can occur in both wealthy and poorer countries. Animal-source foods are the only Prevalence of deficiencies natural source of vitamin B12, so deficiency is prevalent when intake of these foods is low due to their high The recent review by WHO showed that the majority cost, lack of availability, or cultural or religious beliefs. of data on the prevalence of folate and vitamin B12 Deficiency is certainly more prevalent in strict vegetar- deficiencies are derived from relatively small, local ians, but lacto-ovo vegetarians are also at higher risk surveys, but these and national survey data from a for inadequate intakes. If the mother is folate-depleted few countries suggest that deficiencies of both of these during lactation, breastmilk concentrations of the vitamins may be a public health problem that could vitamin are maintained while the mother becomes affect many millions of people throughout the world. -

New Advances in Understanding Iron Deficiency, Treatment and Relationship to Fatigue
New Advances in Understanding Iron Deficiency, Treatment and Relationship to Fatigue Thomas J. Smith, M.D. Center for Cancer and Blood Disorders Objectives 1. Recognize causes of iron deficiency anemia in the pediatric age group, including new understanding of iron deficiency in young athletes. 2. Discuss controversy concerning low ferritin levels and relationship to fatigue. 3. Review possible uses of the new, safer IV iron preparations. 2 Normal Iron Metabolism • meticulous balance between dietary uptake and loss • average adult has 4-5 grams of iron • 1 mg lost each day from sloughing of cells • menstruating females lose an additional 1 mg daily • absorption primary means of regulating iron stores 3 Iron Absorption • primarily in proximal duodenum • increased iron uptake with ascorbate and citrate • phytates, bran and tannins inhibit iron absorption 4 Importance of Iron • indispensible for DNA synthesis and host of metabolic processes • deficiency arrests cell proliferation • most iron ultimately incorporated into hemoglobin • deficiency impairs neurologic function perhaps by effect on neurotransmitters, dopamine receptors, myelination or cytochromes 5 Storage Sites 1. Ferritin – tissue stores correlates with total body iron stores 2. Transferrin – small amount of iron circulates in plasma bound to transferrin. TIBC is sum of iron binding sites on transferrin 3. Hemoglobin – contains 60-80% of total body iron stores 6 Three Stages of Iron Deficiency 1. Prelatent – tissue stores depleted without change in serum iron or hemoglobin. Manifest -

JMSCR Vol||09||Issue||03||Page 21-26||March 2021
JMSCR Vol||09||Issue||03||Page 21-26||March 2021 http://jmscr.igmpublication.org/home/ ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v9i3.05 Scurvy Yes, it exists Authors Samira Aggoune, Hachemi Maouche Departement of Pediatric’s, University Teaching Hospital, Belfort Algier’s. Algeria Pediatrician, Professor Abstract Scurvy is not gone from the face of the planet. In the past it was seen commonly in sailors and soldiers who had limited access to fresh fruits and vegetables over extended periods. Scurvy still exists today within certain populations, particularly in patients with neurodevelopmental disabilities, psychiatric illness or unusual dietary habits. We describe a case of scurvy occurring appeared in a 4-year-old boy with a special diet such as milk and cookies from a young age, presented in a month earlier: asthenia, diffuse lower-extremity musculoskeletal pain, and refusal to walk. Introduction Case Presentation Scurvy stands out in our minds as something that A four year old boy with history of worsening is so basic and easy to avoid, and yet these people musculoskeletal pain and refusal to walk for one have ended up falling victim to an illness that month. His mother had noticed weight loss, simply should not exist in a developed country. lethargy, anorexia, fatigue and weakness. A bone Humans cannot synthesize vitamin C. It needs to story for which he landed in childhood surgery come from external sources, especially fruits and then the patient was referred to us. In his personal vegetables, or fortified foods. antecedents include the notion of six febrile Scurvy can occur in children whose diet is convulsions brought under Depakine restricted as a consequence of developmental or Physical examination disclosed impaired general socioeconomic circumstances[1,2]. -

Cardiac Cachexia: Perspectives for Prevention and Treatment
Review Article Cardiac Cachexia: Perspectives for Prevention and Treatment Marina Politi Okoshi, Rafael Verardino Capalbo, Fernando G Romeiro, Katashi Okoshi Departamento de Clínica Médica - Faculdade de Medicina de Botucatu - Universidade Estadual Paulista - UNESP, São Paulo, SP - Brazil Abstract known as cardiac remodeling. This process is accompanied Cachexia is a prevalent pathological condition associated by cardiac and systemic neurohormonal and inflammatory with chronic heart failure. Its occurrence predicts increased activation, which adversely affects the heart in a vicious 3 morbidity and mortality independent of important clinical cycle and jeopardizes different organs and systems. In recent variables such as age, ventricular function, or heart failure decades, it has become clear that pathological changes functional class. The clinical consequences of cachexia are involve not only the cardiovascular system, but also the dependent on both weight loss and systemic inflammation, renal, neuroendocrinological, immunological, hematologic, which accompany cachexia development. Skeletal muscle gastrointestinal, and musculoskeletal systems, as well as the wasting is an important component of cachexia; it often nutritional status. Currently, experimental and clinical studies precedes cachexia development and predicts poor outcome have focused on the physiopathology of heart failure-related in heart failure. Cachexia clinically affects several organs and systemic complications in order to establish treatments to systems. It is a multifactorial condition where underlying improve quality of life and increase survival. pathophysiological mechanisms are not completely understood Cachexia is a prevalent and important pathological making it difficult to develop specific prevention and treatment condition associated with chronic heart failure. Its occurrence therapies. Preventive strategies have largely focused on muscle predicts reduced survival, independent of relevant variables mass preservation. -
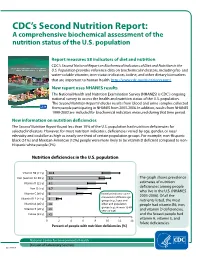
CDC's Second Nutrition Report
CDC’s Second Nutrition Report: A comprehensive biochemical assessment of the nutrition status of the U.S. population Report measures 58 indicators of diet and nutrition CDC’s Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population provides reference data on biochemical indicators, including fat- and water-soluble vitamins, iron-status indicators, iodine, and other dietary biomarkers that are important to human health. http://www.cdc.gov/nutritionreport/ New report uses NHANES results The National Health and Nutrition Examination Survey (NHANES) is CDC’s ongoing national survey to assess the health and nutrition status of the U.S. population. The Second Nutrition Report includes results from blood and urine samples collected from people participating in NHANES from 2003-2006. In addition, results from NHANES 1999-2002 are included for biochemical indicators measured during that time period. New information on nutrition deficiencies The Second Nutrition Report found less than 10% of the U.S. population had nutrition deficiencies for selected indicators. However, for most nutrition indicators, deficiencies varied by age, gender, or race/ ethnicity and could be as high as nearly one third of certain population groups. For example, non-Hispanic black (31%) and Mexican-American (12%) people were more likely to be vitamin D deficient compared to non- Hispanic white people (3%). Nutrition deficiencies in the U.S. population 10.5 9.5 The graph shows prevalence 8.1 estimates of nutrition deficiencies among people 6.7 who live in the U.S. (NHANES 6 Nutrition indicators were 2003-2006). Of all the 2 groups (e.g.,1 year and nutrients listed, the most <1 older) and population people had vitamin B6, iron, groups (e.g., women 12-49 <1 years of age). -

Micronutrient Deficiencies and Stunting Were Associated With
nutrients Article Micronutrient Deficiencies and Stunting Were Associated with Socioeconomic Status in Indonesian Children Aged 6–59 Months Fitrah Ernawati 1, Ahmad Syauqy 2,3,* , Aya Yuriestia Arifin 1, Moesijanti Y. E. Soekatri 4 and Sandjaja Sandjaja 5 1 Center of Research and Development for Biomedical and Basic Technology of Health, National Institute of Health Research and Development, Jakarta 10560, Indonesia; fi[email protected] (F.E.); ayarifi[email protected] (A.Y.A.) 2 Department of Nutrition Science, Faculty of Medicine, Diponegoro University, Jawa Tengah 50275, Indonesia 3 Center of Nutrition Research (CENURE), Diponegoro University, Jawa Tengah 50275, Indonesia 4 Nutrition Department, Health Polytechnic Ministry of Health of Jakarta II, Jakarta 12120, Indonesia; [email protected] 5 Persatuan Ahli Gizi (PERSAGI), Jakarta 12320, Indonesia; [email protected] * Correspondence: [email protected]; Tel./Fax: +62-24-76402881 Abstract: Micronutrient deficiencies and stunting are known as a significant problem in most de- veloping countries, including Indonesia. The objective of this study was to analyze the association between micronutrient deficiencies and stunting with socioeconomic status (SES) among Indonesian children aged 6–59 months. This cross-sectional study was part of the South East Asian Nutrition Surveys (SEANUTS). A total of 1008 Indonesian children were included in the study. Anemia, iron deficiency, vitamin A deficiency, vitamin D deficiency, and stunting were identified in this study. Structured questionnaires were used to measure SES. Differences between micronutrient parameters Citation: Ernawati, F.; Syauqy, A.; and anthropometric indicators with the SES groups were tested using one-way ANOVA with post- Arifin, A.Y.; Soekatri, M.Y.E.; Sandjaja, hoc test after adjusted for age, area resident (rural and urban), and sex. -

Iron Nutrition During the First 6 Months of Life
Ensuring a healthy start for future development: Iron nutrition during the fi rst 6 months of life The importance of iron for healthy The magnitude of iron defi ciency development Figure 1: Prevalence of anemia (hemoglobin < 11 g/dL) among Iron is an essential nutrient for optimal mental, motor and 6 to 11 month-old children in countries in Latin behavioral development. It is present in all body cells and America and the Caribbean is fundamental for basic physiological processes such as hemoglobin production and enzyme function. Iron defi ciency 80 (ID) generally results when dietary iron intake cannot meet 70 required needs and iron reserves become depleted. Because 60 iron needs are highest during periods of rapid growth or 50 when frequent blood loss occurs (e.g. through menstruation or intestinal bleeding), infants and children, pregnant women 40 anemia % and women of reproductive age are at high risk of developing Prevalence of 30 ID. 20 Like other micronutrient defi ciencies, ID is generally not 10 outwardly apparent, even though it may already be negatively 0 3 5 4 2 4 '0 '0 05 '0 '0 '0 aff ecting fundamental physiological processes. In its most ia ití s ' ru la or liv a ra e a ad o H du P tem lv B on ua Sa advanced stage, when iron stores have been depleted, anemia H G El develops. Anemia is defi ned as insuffi cient hemoglobin or red blood cells. Anemia caused by ID is referred to as iron 6-9 months 6-11 months defi ciency anemia (IDA). -

Iron Deficiency Anemia: a Common and Curable Disease
Downloaded from http://perspectivesinmedicine.cshlp.org/ at University of Buenos Aires on May 14, 2013 - Published by Cold Spring Harbor Laboratory Press Iron Deficiency Anemia: A Common and Curable Disease Jeffery L. Miller Cold Spring Harb Perspect Med published online April 23, 2013 Subject Collection Hemoglobin and Its Diseases Cell-Free Hemoglobin and Its Scavenger Proteins: Clinical Manifestations of α-Thalassemia New Disease Models Leading the Way to Targeted Elliott P. Vichinsky Therapies Dominik J. Schaer and Paul W. Buehler The Molecular Basis of β-Thalassemia Iron Deficiency Anemia: A Common and Curable Swee Lay Thein Disease Jeffery L. Miller Erythroid Heme Biosynthesis and Its Disorders Erythropoiesis: Development and Differentiation Harry A. Dailey and Peter N. Meissner Elaine Dzierzak and Sjaak Philipsen Hemoglobin Variants: Biochemical Properties and Erythropoietin Clinical Correlates H. Franklin Bunn Christopher S. Thom, Claire F. Dickson, David A. Gell, et al. The Prevention of Thalassemia Classification of the Disorders of Hemoglobin Antonio Cao and Yuet Wai Kan Bernard G. Forget and H. Franklin Bunn The Switch from Fetal to Adult Hemoglobin The Molecular Basis of α-Thalassemia Vijay G. Sankaran and Stuart H. Orkin Douglas R. Higgs Pathophysiology and Clinical Manifestations of Evolution of Hemoglobin and Its Genes the β-Thalassemias Ross C. Hardison Arthur W. Nienhuis and David G. Nathan Development of Gene Therapy for Thalassemia The Search for Genetic Modifiers of Disease Arthur W. Nienhuis and Derek A. Persons Severity in the β-Hemoglobinopathies Guillaume Lettre For additional articles in this collection, see http://perspectivesinmedicine.cshlp.org/cgi/collection/ Copyright © 2013 Cold Spring Harbor Laboratory Press; all rights reserved Downloaded from http://perspectivesinmedicine.cshlp.org/ at University of Buenos Aires on May 14, 2013 - Published by Cold Spring Harbor Laboratory Press Iron Deficiency Anemia: A Common and Curable Disease Jeffery L.