Hepatitis C W1-W8
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regions Hospital Delineation of Privileges Surgery
Regions Hospital Delineation of Privileges Surgery Applicant’s Name: ____________________________________________________________________________ Last First M. Instructions: Place a check-mark where indicated for each core group you are requesting. Review education and basic formal training requirements to make sure you meet them. Review documentation and experience requirements and be prepared to prove them. Note all renewing applicants are required to provide evidence of their current ability to perform the privileges being requested\ When documentation of cases or procedures is required, attach said case/procedure logs to this privileges-request form. Provide complete and accurate names and addresses where requested -- it will greatly assist how quickly our credentialing-specialist can process your requests. Overview: (Applicant should check all core privileges you are requesting) Core I – General Staff Privileges in Surgery Core II – General Staff Privileges in Trauma (Adult and Pediatric) Core III – Pediatric Trauma Rounding Privileges Core IV – General Staff Privileges in Burn Core V – General Staff Privileges in Colon and Rectal Surgery Core VI – General Staff Privileges in Vascular Surgery Core VII – General Staff Privileges in Surgical Critical Care Special Privileges Also included are: Core Procedure Lists Signature Page Page 1 of 24 06.2015 CORE I -- General Staff Privileges in Surgery (Appointments are based on the needs of the Department of Surgery as determined by the Division Head of Surgery and Hospital Board) Privileges Privileges include the performance of surgical procedures (including related admission, consultation, work-up, pre- and post-operative care) to correct or treat various conditions, illnesses and injuries of the: alimentary tract, including colon and rectum, abdomen and its contents, breasts, skin, and soft tissue, head and neck, endocrine system and vascular system, excluding the intercranial vessels, the heart and those vessels intrinsic and immediately adjacent thereto. -

1311 Diploma in Medical Record Science Second
[LD 0212] AUGUST 2013 Sub. Code: 1311 DIPLOMA IN MEDICAL RECORD SCIENCE SECOND YEAR PAPER II – INTERNATIONAL CLASSIFICATION OF DISEASES (ICD-10) & SURGICAL PROCEDURES (ICM-9CM) Q.P. Code : 841311 Time : Three Hours Maximum : 100 marks Answer ALL questions I Write appropriate codes using ICD -10 (30 x 1 = 30) 1. Therapeutic introduction of hand tendon. 2. Excision of major partial thickness of eyelid excision. 3. Interphalangeal arthrodesis of Toe. 4. Division of percutaneous spinal cord nerve tracts. 5. Transfusion of allograft bone aetriosus. 6. Rastelli operation of truncus arteriosus. 7. Pyoloric sphincter dilatation. 8. Stapling of radius epiphyseal plate. 9. Suture of hands fascia. 10. Suture of hand fascia. 11. Repair of anterior wall (abdomen) hernia. 12. Foreign body removal without incision in t o the brain. 13. Repair of Tetrology of fallot. 14. Frontal Sinusectomy. 15. Urethral sling suspension. 16. Bone shaft transfer. 17. Coil of aneuryum repair. 18. Sling suspension. 19. Radio isotope scanning, pituitary gland. 20. Spinal shunt removal. 21. Acute lung edema. Due to external agent. 22. Proximal end tibial closed fracture was riding a two wheeler-slip & fell down. 23. Thrombosed internal hemorrhoids. 24. Secondary hypertension due to renal disorder. 25. Old myocardial infarction. 26. Fall from high place, injured elbow. 27. Chronic venous (peripheral) insufficiency. 28. Acute myeloid leukemia. 29. Post-operative intestine obstruction. 30. Abnormal pregnancy. II Writes appropriate codes using ICS-9CM (20 x 2 = 40) 1. Pregnant women suffering from acute salphingo oophoritis. 2. Accidental intake of ferrous salt. 3. Sprain of lumbar spine as stuck by another person. 4. -

Anal Cancer Anal Cancer, Also Known As Anal Carcinoma, Is Cancer of the Anus
Anal Cancer Anal cancer, also known as anal carcinoma, is cancer of the anus. To help diagnose this condition, your doctor will perform a digital rectal exam and anoscopy. An MRI, CT, PET/CT, or an endoanal ultrasound may also be ordered by your doctor. Depending on the size, location, and extent of the cancer, treatments may include surgery, radiation therapy and chemotherapy. What is anal cancer? Anal cancer is a cancer that begins in the anus, the opening at the end of the gastrointestinal tract through which stool, or solid waste, leaves the body. The anus begins at the bottom of the rectum, which is the last part of the large intestine (also called the colon). Anal cancer usually affects adults over age 60 and women more often than men. More than 8,000 people in the U.S. are diagnosed with anal cancer each year. Anal cancer symptoms may include changes in bowel habits and changes in and around the anal area, including: bleeding and itching pain or pressure unusual discharge a lump or mass fecal incontinence fistulae. Some patients with anal cancers do not experience any symptoms. Some non-cancerous conditions, such as hemorrhoids and fissures, may cause similar symptoms. How is anal cancer diagnosed and evaluated? To diagnose the cause of symptoms, your doctor may perform: Digital rectal examination (DRE): Digital Rectal Exam (DRE): This test examines the lower rectum and the prostate gland in males to check for abnormalities in size, shape or texture. The term "digital" refers to the clinician's use of a gloved lubricated finger to conduct the exam. -

Is Digital Rectal Exam Reliable in Grading Anal Sphincter Defects?
DOI: 10.1590/S0004-28032016000400006 ARQGA/1864 IS DIGITAL RECTAL EXAM RELIABLE IN GRADING ANAL SPHINCTER DEFECTS? Marcelo de Melo Andrade COURA, Silvana Marques SILVA, Romulo Medeiros de ALMEIDA, Miles Castedo FORREST and João Batista SOUSA Received 7/7/2015 Accepted 14/8/2015 ABSTRACT - Background - Anal sphincter tone is routinely assessed by digital rectal examination in patients with fecal incontinence, although its accuracy in detecting sphincter defects or separating competent from incompetent muscles has not been established. Objective - In this setting, we aimed to evaluate the accuracy of digital rectal examination in grading anal defects in order to sepa- rate small from extensive cases as depicted on 3D endoanal ultrasound, using a scoring sphincter defect and correlate anal tone to anal pressures. Methods - Women with fecal incontinence were divided into two groups: small or extensive defects according to the ultrasound scoring system. Sensitivity, specificity, positive and negative predictive values of digital rectal examination in grading global and external sphincter defects were calculated. Anal tone at digital rectal examination was compared to resting and incremen- tal pressures. Results - A cohort of 76 consecutive incontinent women were enrolled. The median Wexner score was 9. Sixty-eight showed sphincter defects on 3D endoanal ultrasound. Anal tone at digital rectal examination was considered abnormal in 62 cases. Abnormal digital rectal examination showed a sensitivity of 90%, specificity of 27.78% in distinguishing small from extensive defects of both sphincters. Five out of eight women with no sphincter defects had only abnormal squeeze tone at digital rectal examination. Abnormal squeeze tone at digital rectal examination had a sensitivity of 65.31% in distinguishing small from extensive external anal sphincter defects. -

Endorectal, Endoanal and Perineal Ultrasound
Review Nuernberg Dieter et al. EFSUMB Recommendations for Gastrointestinal … Ultrasound Int Open 2018; 00: 00–00 EFSUMB Recommendations for Gastrointestinal Ultrasound Part 3: Endorectal, Endoanal and Perineal Ultrasound Authors Dieter Nuernberg1,*, Adrian Saftoiu2,*, Ana Paula Barreiros3, Eike Burmester4, Elena Tatiana Ivan2, Dirk-André Clevert5, Christoph F. Dietrich6, Odd Helge Gilja7, Torben Lorentzen8, Giovanni Maconi9, Ismail Mihmanli10, Christian Pallson Nolsoe11, Frank Pfeffer12, Søren Rafael Rafaelsen13, Zeno Sparchez14, Peter Vilmann15, Jo Erling Riise Waage12 Affiliations Key words 1 Medical School Brandenburg Theodor Fontane, endorectal ultrasound, endoanal ultrasound, perineal ultrasound Gastroenterology, Neuruppin, Germany received 07.04.2018 2 Research Center in Gastroenterology and Hepatology, revised 23.11.2018 University of Medicine and Pharmacy Craiova, Craiova, accepted 01.12.2018 Romania 3 Deutsche Stiftung Organtransplantation, Head of Bibliography Organisation Center Middle, Frankfurt, Germany DOI https://doi.org/10.1055/a-0825-6708 4 Department of Internal Medicine/Gastroenterology, Published online: 2019 Sana-Kliniken Lübeck, Lübeck, Germany Ultrasound Int Open 2019; 5: E34–E51 5 Department of Clinical Radiology, Interdisciplinary © Georg Thieme Verlag KG Stuttgart · New York Ultrasound-Center, University of Munich-Grosshadern ISSN 2199-7152 Campus, Munich, Germany 6 Caritas-Krankenhaus, Medizinische Klinik 2, Bad Correspondence Mergentheim, Germany Prof. Adrian Saftoiu 7 National Centre for Ultrasound in Gastroenterology, -

Obstetric Anal Sphincter Injury Detection Using Impedance Spectroscopy with the ONIRY Probe
applied sciences Article Obstetric Anal Sphincter Injury Detection Using Impedance Spectroscopy with the ONIRY Probe Marcel Mły ´nczak 1 , Maciej Rosoł 1 , Antonino Spinelli 2,3 , Adam Dziki 4 , Edyta Wla´zlak 5 , Grzegorz Surkont 5 , Magda Krzycka 5 , Paulina Paj ˛ak 5 , Łukasz Dziki 4 , Michał Mik 4 and Katarzyna Borycka-Kiciak 6,* 1 Faculty of Mechatronics, Institute of Metrology and Biomedical Engineering, Warsaw University of Technology, 02-525 Warsaw, Poland; [email protected] (M.M.); [email protected] (M.R.) 2 Humanitas Clinical and Research Center—IRCCS, 20089 Milan, Italy; [email protected] 3 Department of Biomedical Sciences, Humanitas University, 20090 Milan, Italy 4 Department of General and Colorectal Surgery, Medical University of Lodz, 90-647 Lodz, Poland; [email protected] (A.D.); [email protected] (Ł.D.); [email protected] (M.M.) 5 First Department of Gynecology and Obstetrics, Clinic for Gynecological Surgery and Oncology, Medical University of Lodz, 94-029 Lodz, Poland; [email protected] (E.W.); [email protected] (G.S.); [email protected] (M.K.); [email protected] (P.P.) 6 Centre of Postgraduate Medical Education, Department of Colorectal, General and Oncological Surgery, 01-813 Warsaw, Poland * Correspondence: [email protected] Featured Application: Impedance spectroscopy performed using ONIRY can be used to screen for possible obstetric anal sphincter injury just after natural delivery, allowing the start of necessary treatment or rehabilitation. Citation: Mły´nczak,M.; Rosoł, M.; Abstract: Anal sphincter injuries occurring during natural deliveries are often a reason for severe Spinelli, A.; Dziki, A.; Wla´zlak,E.; Surkont, G.; Krzycka, M.; Paj ˛ak,P.; complications, including fecal incontinence. -
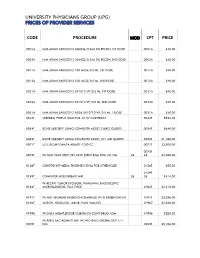
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Endorectal, Endoanal and Perineal Ultrasound
University of Southern Denmark EFSUMB Recommendations for Gastrointestinal Ultrasound Part 3 Endorectal, Endoanal and Perineal Ultrasound Nuernberg, Dieter; Saftoiu, Adrian; Barreiros, Ana Paula; Burmester, Eike; Ivan, Elena Tatiana; Clevert, Dirk-André; Dietrich, Christoph F; Gilja, Odd Helge; Lorentzen, Torben; Maconi, Giovanni; Mihmanli, Ismail; Nolsoe, Christian Pallson; Pfeffer, Frank; Rafaelsen, Søren Rafael; Sparchez, Zeno; Vilmann, Peter; Waage, Jo Erling Riise Published in: Ultrasound International Open DOI: 10.1055/a-0825-6708 Publication date: 2019 Document version: Final published version Document license: CC BY-NC-ND Citation for pulished version (APA): Nuernberg, D., Saftoiu, A., Barreiros, A. P., Burmester, E., Ivan, E. T., Clevert, D-A., Dietrich, C. F., Gilja, O. H., Lorentzen, T., Maconi, G., Mihmanli, I., Nolsoe, C. P., Pfeffer, F., Rafaelsen, S. R., Sparchez, Z., Vilmann, P., & Waage, J. E. R. (2019). EFSUMB Recommendations for Gastrointestinal Ultrasound Part 3: Endorectal, Endoanal and Perineal Ultrasound. Ultrasound International Open, 5(1), E34-E51. https://doi.org/10.1055/a- 0825-6708 Go to publication entry in University of Southern Denmark's Research Portal Terms of use This work is brought to you by the University of Southern Denmark. Unless otherwise specified it has been shared according to the terms for self-archiving. If no other license is stated, these terms apply: • You may download this work for personal use only. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying this open access version If you believe that this document breaches copyright please contact us providing details and we will investigate your claim. -

Anal Fissure
Anal Fissure What is an anal fissure? An anal fissure is a small tear in the skin of the anus. The anus is the opening of the rectum where bowel movements (BMs) leave the body. Anal fissures are a fairly common problem. What is the cause? A tear may happen when you have: • Hard, dry bowel movements • Constipation • Hemorrhoids • Anal surgery • Inflammation of the rectum caused by intestinal problems such as Crohn's disease What are the symptoms? Symptoms may include: • Pain during or after bowel movements • Cramping of the muscle at the opening of the anus caused by irritation of the tear during a bowel movement • Bright red blood when you have a bowel movement (you may see the blood on the BM, in the toilet water, or on toilet tissue you have used) How is it diagnosed? Your healthcare provider will ask about your symptoms and examine you. You may have a procedure called an anoscopy to confirm the diagnosis. For this procedure your provider uses a small tool with a light called an anoscope to examine the anus and lower part of the rectum. Your healthcare provider may recommend other tests or procedures, such as a sigmoidoscopy or colonoscopy, to learn more about the cause of the fissure or the bleeding. How is it treated? Most fissures will heal with the following at-home treatments: • Your healthcare provider may recommend a stool softener, such as Haley's M-O, psyllium, Metamucil or Citrucel, or mineral oil. • It may help to drink lots of water and add more fiber to your diet. -

Sacral Neuromodulation System Neurostimulator Implant Manual
Sacral Neuromodulation System Neurostimulator Implant Manual Model 1101 Neurostimulator Rx only Axonics®, Axonics Modulation®, Axonics Modulation Technologies® and Axonics Sacral Neuromodulation System® are trademarks of Axonics Modulation Technologies, Inc., registered or pending registration in the U.S. and other countries. Axonics Modulation Technologies, Inc. 26 Technology Drive Irvine, CA 92618 (USA) www.axonicsmodulation.com Tel. +1-877-929-6642 Fax +1-949 396-6321 LABEL SYMBOLS This section explains the symbols found on the product and packaging. Symbol Description Symbol Description Refer to instructions for use (Consult accompanying documents) Axonics Neurostimulator Temperature limitation Axonics Torque Wrench Humidity limitation Neurostimulator default waveform with 14 Hz frequency, 0 mA Pressure limitation amplitude and 210 ms pulse width Do not reuse Neurostimulator default electrode configuration: Electrode 0: Negative (-) Electrode 1: Off (0) Sterilized using Ethylene oxide Electrode 2: Off (0) Electrode 3: Positive (+) Use by Case: Off (0) Authorized representative in the European community Open here Do not use if package is damaged Product Serial Number Do not resterilize For USA audiences only Manufacturer !USA Rx ONLY Caution: U.S. Federal law restricts this device for sale by or on the order of a physician Product Model Number Warning / Caution Manufacturing Date Product Literature Non ionizing electromagnetic radiation Magnetic Resonance (MR) Conditional FCC ID US Federal Communications Commission device identification IC Industry Canada certification number Conformité Européenne (European Conformity). This symbol means This device complies with all applicable Australian Communica- that the device fully complies with AIMD Directive 90/385/EEC R-NZtions and Media Authority (ACMA) regulatory arrangements and (Notified Body reviewed) and RED 2014/53/EU (self-certified) electrical equipment safety requirements 3 TABLE OF CONTENTS LABEL SYMBOLS . -

Evaluation of Diagnostic Accuracy of 3-D Endoanal Ultrasound In
j coloproctol (rio j). 2 0 1 8;3 8(1):9–12 Journal of Coloproctology www.jcol.org.br Original Article Evaluation of diagnostic accuracy of 3-D endoanal ultrasound in perianal fistula in comparison with intraoperative findings a a,∗ b c Ehsan Rahmanian , Mojgan Frootan , Fakhri Anaraki , Elahe Alimadadi , d Mahsa Ramezanpoor a Shahid Beheshti of Medical Sciences, Tehran, Iran b Ayatollah Taleghani Hospital, Surgery Department, Tehran, Iran c Shahid Beheshti of Medical Sciences, Research Institute for Gastroenterology and Liver Diseases, Tehran, Iran d Shahid Sadoughi University of Medical Sciences, Research Center – Treatment of Diabetes, Tehran, Iran a r t a b i c s t l e i n f o r a c t Article history: Objective: Perianal fistula is a common and debilitating disease. The definite treatment is Received 24 May 2017 surgery, identifying of primary and secondary tract, internal opening of fistula has important Accepted 26 August 2017 role in planning of surgical techniques. This study’s goal was to determine the diagnostic Available online 26 November 2017 accuracy of 3-D ultrasound in perianal fistula in comparison with intraoperative findings. Materials and methods: This study is a cross-sectional study. Adult patients (18–85 years old) Keywords: with anal fistula have been selected. 3-D EUS was done for all patients by gastroenterologist. Then surgery was done. Check lists filled by endoscopist and surgeon was studied and data Anal fistula Three dimensional endoscopic analysis was done. ultrasounds Results: The study examined 76 patients, in according to results for kappa coefficient there Surgery was a perfect agreement between 3-D ultrasound results and surgery in internal opening that was 96% (p < 0.001) and concordance was 0.974. -

PSI Appendix a Version 6.0 Patient Safety Indicators Appendices
AHRQ QI™ ICD-9-CM Specification Version 6.0 Patient Safety Indicators Appendices www.qualityindicators.ahrq.gov APPENDIX A: Operating Room Procedure Codes Operating room procedure codes: ORPROC_PSI 0050 IMPL CRT PACEMAKER SYS 4972 ANAL CERCLAGE 0051 IMPL CRT DEFIBRILLAT SYS 4973 CLOSURE OF ANAL FISTULA 0052 IMP/REP LEAD LF VEN SYS 4974 GRACILIS MUSC TRANSPLAN 0053 IMP/REP CRT PACEMAKR GEN 4975 IMPL OR REV ART ANAL SPH 0054 IMP/REP CRT DEFIB GENAT 4976 REMOV ART ANAL SPHINCTER 0056 INS/REP IMPL SENSOR LEAD 4979 ANAL SPHINCT REPAIR NEC 0057 IMP/REP SUBCUE CARD DEV 4991 INCISION OF ANAL SEPTUM 0061 PERC ANGIO PRECEREB VESS 4992 INSERT SUBQ ANAL STIMUL 0062 PERC ANGIO INTRACRAN VES 4993 ANAL INCISION NEC 0066 PTCA OR CORONARY ATHER 4994 REDUCTION ANAL PROLAPSE 0070 REV HIP REPL-ACETAB/FEM 4995 CONTROL ANAL HEMORRHAGE 0071 REV HIP REPL-ACETAB COMP 4999 ANAL OPERATION NEC 0072 REV HIP REPL-FEM COMP 500 HEPATOTOMY 0073 REV HIP REPL-LINER/HEAD 5012 OPEN LIVER BIOPSY 0080 REV KNEE REPLACEMT-TOTAL 5014 LAPAROSCOPIC LIVER BX 0081 REV KNEE REPL-TIBIA COMP 5019 HEPATIC DX PROC NEC 0082 REV KNEE REPL-FEMUR COMP 5021 MARSUPIALIZAT LIVER LES 0083 REV KNEE REPLACE-PATELLA 5022 PARTIAL HEPATECTOMY 0084 REV KNEE REPL-TIBIA LIN 5023 OPN ABLTN LIVER LES/TISS 0085 RESRF HIPTOTAL-ACET/FEM 5024 PERC ABLTN LIVER LES/TIS 0086 RESRF HIPPART-FEM HEAD 5025 LAP ABLTN LIVER LES/TISS 0087 RESRF HIPPART-ACETABLUM 5026 ABLTN LIVER LES/TISS NEC 0112 OPEN CEREB MENINGES BX 5029 DESTRUC HEPATIC LES NEC 0114 OPEN BRAIN BIOPSY 503 HEPATIC LOBECTOMY 0115 SKULL BIOPSY 504 TOTAL