1 Online Supplement: Association of Asthma Severity And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Additions and Deletions to the Drug Product List
Prescription and Over-the-Counter Drug Product List 40TH EDITION Cumulative Supplement Number 09 : September 2020 ADDITIONS/DELETIONS FOR PRESCRIPTION DRUG PRODUCT LIST ACETAMINOPHEN; BUTALBITAL; CAFFEINE TABLET;ORAL BUTALBITAL, ACETAMINOPHEN AND CAFFEINE >A> AA STRIDES PHARMA 325MG;50MG;40MG A 203647 001 Sep 21, 2020 Sep NEWA ACETAMINOPHEN; CODEINE PHOSPHATE SOLUTION;ORAL ACETAMINOPHEN AND CODEINE PHOSPHATE >D> AA WOCKHARDT BIO AG 120MG/5ML;12MG/5ML A 087006 001 Jul 22, 1981 Sep DISC >A> @ 120MG/5ML;12MG/5ML A 087006 001 Jul 22, 1981 Sep DISC TABLET;ORAL ACETAMINOPHEN AND CODEINE PHOSPHATE >A> AA NOSTRUM LABS INC 300MG;15MG A 088627 001 Mar 06, 1985 Sep CAHN >A> AA 300MG;30MG A 088628 001 Mar 06, 1985 Sep CAHN >A> AA ! 300MG;60MG A 088629 001 Mar 06, 1985 Sep CAHN >D> AA TEVA 300MG;15MG A 088627 001 Mar 06, 1985 Sep CAHN >D> AA 300MG;30MG A 088628 001 Mar 06, 1985 Sep CAHN >D> AA ! 300MG;60MG A 088629 001 Mar 06, 1985 Sep CAHN ACETAMINOPHEN; HYDROCODONE BITARTRATE TABLET;ORAL HYDROCODONE BITARTRATE AND ACETAMINOPHEN >A> @ CEROVENE INC 325MG;5MG A 211690 001 Feb 07, 2020 Sep CAHN >A> @ 325MG;7.5MG A 211690 002 Feb 07, 2020 Sep CAHN >A> @ 325MG;10MG A 211690 003 Feb 07, 2020 Sep CAHN >D> AA VINTAGE PHARMS 300MG;5MG A 090415 001 Jan 24, 2011 Sep DISC >A> @ 300MG;5MG A 090415 001 Jan 24, 2011 Sep DISC >D> AA 300MG;7.5MG A 090415 002 Jan 24, 2011 Sep DISC >A> @ 300MG;7.5MG A 090415 002 Jan 24, 2011 Sep DISC >D> AA 300MG;10MG A 090415 003 Jan 24, 2011 Sep DISC >A> @ 300MG;10MG A 090415 003 Jan 24, 2011 Sep DISC >D> @ XIROMED 325MG;5MG A 211690 -

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

Neonatal Intensive Care Drug Therapy Update: a Bibliography
LWW/JPNN AS310-13 July 28, 2004 23:11 Char Count= 0 J Perinat Neonat Nurs Vol. 18, No. 3, pp. 292–306 c 2004 Lippincott Williams & Wilkins, Inc. Neonatal Intensive Care Drug Therapy Update: A Bibliography Jason Sauberan, PharmD BIBLIOGRAPHY I. Overview A. Clark RH, Bloom BT, Gerstmann DR Medications Used in Neonatal Intensive Care Units—A Descriptive Study [abstract 3047]. In: Program and abstracts of the 2004 Pediatric Academic Societies’ Annual Meeting, San Francisco, CA. B. Barr J, Brenner-Zada G, Heiman E, Pareth G, Bulkowstein M, Greenberg R, Berkovitch M. Unlicensed and off-label medication use in a neonatal intensive care unit: a prospective study. Am J Perinatol. 2002 Feb;19(2):67–72. C. O’Donnell CP, Stone RJ, Morley CJ. Unlicensed and off-label drug use in an Australian neonatal intensive care unit. Pediatrics. 2002 Nov;110(5):e52. D. Committee on Drugs. American Academy of Pediatrics. Uses of drugs not described in the package insert (off-label uses). Pediatrics. 2002 Jul;110(1 Pt 1):181–3. II. Anti-infectives A. Linezolid 1. Deville JG, Adler S, Azimi PH, Jantausch BA, Morfin MR, Beltran S, Edge-Padbury B, Naberhuis-Stehouwer S, Bruss JB. Linezolid versus vancomycin in the treatment of known or suspected resistant gram-positive infections in neonates. Pediatr Infect Dis J. 2003 Sep;22(9 Suppl):S158–63. 2. Vo M, Cirincione BB, Rubino CM, Jungbluth GL. Pharmacokinetics of Linezolid in Neonates and Young Infants [abstract A-1409]. In: Program and abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA. -

Effect of Theophylline and Enprofylline on Bronchial Hyperresponsiveness
Thorax: first published as 10.1136/thx.44.12.1022 on 1 December 1989. Downloaded from Thorax 1989;44:1022-1026 Effect of theophylline and enprofylline on bronchial hyperresponsiveness G H KOETER, J KRAAN, M BOORSMA, J H G JONKMAN, TH W VAN DER MARK From the Department ofPulmonology and Lung Function, University Hospital, Groningen; Pharma Bio Research, Assen; and Astra Pharmaceutica, Rijswijk, The Netherlands ABSTRACT The effect of increasing intravenous doses of theophylline and enprofylline, a new xanthine derivative, on bronchial responsiveness to methacholine was studied in eight asthmatic patients. Methacholine provocations were carried out on three days before and after increasing doses of theophylline, enprofylline, and placebo, a double blind study design being used. Methacholine responsiveness was determined as the provocative concentration of methacholine causing a fall of 20% in FEV, (PC20). The patients were characterised pharmacokinetically before the main study to provide an individual dosage scheme for each patient that would provide rapid steady state plasma concentration plateaus of 5, 10, and 15 mg/l for theophylline and 1 25, 2 5, and 3-75 mg/l for enprofylline. Dose increments in the main study were given at 90 minute intervals. FEV, showed a small progressive decrease after placebo; it remained high in relation to placebo after both drugs and this effect was dose related. Methacholine PC20 values decreased after placebo; mean values were (maximum difference 2-0 and 1 7 higher after theophylline and enprofylline than after placebo copyright. doubling doses of methacholine); the effect of both drugs was dose related. Thus enprofylline and theophylline when given intravenously cause a small dose related increase in FEV1 and methacholine PC20 when compared with placebo. -

Application and Review of Pediatric Pharmacotherapy, Sample Chapter
Application and Review of Pediatric Pharmacotherapy Chapter No. 1 Dated: 29/7/2010 At Time: 16:16:4 Section 1 Neonatal intensive care “Luck is where preparation meets opportunity.” This section consists of 16 medication orders followed by corresponding patient profiles representing pharmacotherapy associated with patients admit- ted to a neonatal intensive care unit. Each patient profile is followed by multiple-choice questions pertaining to the medication order and profile infor- mation. Choose the one best-lettered response to each item. The correct answers are provided at the end of this section. The reader is encouraged to attempt all questions for each case or for the entire section prior to referring to the answers. Moreover, where appropriate, the answer key provides a thor- ough explanation of the correct response and should serve as an additional learning tool for the reader. Application and Review of Pediatric Pharmacotherapy Chapter No. 1 Dated: 29/7/2010 At Time: 16:16:4 2 | Application and Review of Pediatric Pharmacotherapy Medication orders Physician order Patient weight: 2.5 kg Aminophylline 10 mg iv load, then begin 2.5 mg iv q 12 h Obtain theophylline concentration 1 h postinfusion of loading dose Date/time: 12/01/2100 Patient name: Baby Boy Turner Physician: John Craver Patient ID: 111222 Medical profile Patient: Baby Boy Turner Patient weight: 2.5 kg Age: 1d/o Present illness: Apneic episodes Allergies: None Medical history: 33 weeks gestation, Apgar 7 and 9 Labs: pending Medication profile Questions Q1 Which of the following is an acceptable definition of apnea of prematurity? 1 o cessation of breathing for less than 20 s 2 o cessation of breathing for at least 20 s 3 o cessation of breathing for less than 20 s when accompanied by bradycardia A o 1 only B o 3 only C o 1 and 3 only Application and Review of Pediatric Pharmacotherapy Chapter No. -

Caffeine in the Treatment of Pain
Rev Bras Anestesiol ARTIGOS DE REVISÃO 2012; 62: 3: 387-401 ARTIGOS DE REVISÃO Cafeína para o Tratamento de Dor Cristiane Tavares, TSA 1, Rioko Kimiko Sakata, TSA 2 Resumo: Tavares C, Sakata RK – Cafeína para o Tratamento de Dor. Justificativa e objetivos: A cafeína é uma substância amplamente consumida com efeitos em diversos sistemas e que apresenta farmacoci- nética e farmacodinâmica características, causando interações com diversos medicamentos. O objetivo deste estudo é fazer uma revisão sobre os efeitos da cafeína. Conteúdo: Nesta revisão, são abordados a farmacologia da cafeína, os mecanismos de ação, as indicações, as contraindicações, as doses, as interações e os efeitos adversos. Conclusões: Faltam estudos controlados, randomizados e duplos-cegos para avaliar a eficácia analgésica da cafeína nas diversas síndromes dolorosas. Em pacientes com dor crônica, é necessário ter cautela em relação ao desenvolvimento de tolerância, abstinência e interação medi- camentosa no uso crônico de cafeína. Unitermos: ANALGESIA; DOR; DROGAS, Alcaloide/cafeína. ©2012 Elsevier Editora Ltda. Todos os direitos reservados. INTRODUÇÃO Estrutura química A cafeína foi isolada em 1820, mas a estrutura correta des- A cafeína é um alcaloide presente em mais de 60 espécies ta metilxantina foi estabelecida na última década do século de plantas 4. Sua estrutura molecular pertence a um grupo XIX. Os efeitos não foram claramente reconhecidos até 1981, de xantinas trimetiladas que incluem seus compostos inti- quando o bloqueio de receptores adenosina foi correlacio- mamente relacionados: teobromina (presente no cacau) e nado às propriedades estimulantes da cafeína e de seus teofilina (presente no chá) 1. Quimicamente, esses alcaloides análogos 1. Provavelmente a cafeína é uma das substâncias são semelhantes a purinas, xantinas e ácido úrico, que são psicoativas mais utilizadas no mundo, promovendo efeitos compostos metabolicamente importantes 4. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -
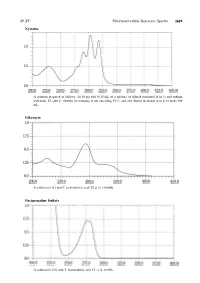
Nystatin Ofloxacin Orciprenaline Sulfate
JP XV Ultraviolet-visible Reference Spectra 1619 Nystatin A solution prepared as follows: To 10 mg add 50.25 mL of a mixture of diluted methanol (4 in 5) and sodium hydroxide TS (200:1), dissolve by warming at not exceeding 509C,andadddilutedmethanol(4in5)tomake500 mL. Ofloxacin Asolutionin0.1mol/LhydrochloricacidTS(1in150,000) Orciprenaline Sulfate A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) 1620 Ultraviolet-visible Reference Spectra JP XV Oxazolam A solution in ethanol (95) (1 in 100,000) Oxethazaine A solution in ethanol (95) (1 in 2500) Oxybuprocaine Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1621 Oxycodone Hydrochloride Hydrate An aqueous solution (1 in 10,000) Oxymetholone A solution prepared as follows: To 5 mL of a solution in methanol (1 in 5000) add 5 mL of sodium hydroxide-methanol TS and methanol to make 50 mL. Oxytetracycline Hydrochloride Asolutionin0.1mol/LhydrochloricacidTS(1in50,000) 1622 Ultraviolet-visible Reference Spectra JP XV Oxytocin An aqueous solution (1 in 2000) Penbutolol Sulfate A solution in methanol (1 in 10,000) Pentazocine A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) JP XV Ultraviolet-visible Reference Spectra 1623 Peplomycin Sulfate Asolutionpreparedasfollows:To4mgadd5mL of copper (II) sulfate TS, and dissolve in water to make 100 mL. Perphenazine 1 Asolutionin0.1mol/LhydrochloricacidTS(1in200,000) Perphenazine 2 A solution obtained by adding 10 mL of water to 10 mL of the solution for Perphenazine 1 1624 Ultraviolet-visible -

Caffeine Citrate 10Mg/Ml Oral Solution Maintenance Doses
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT doctor or nurse may give one more higher • changes in blood tests (reduced levels dose, before continuing to the lower of haemoglobin after prolonged Caffeine Citrate 10mg/ml Oral Solution maintenance doses. treatment) Duration of treatment • reduced thyroid hormone at the start Equivalent to Caffeine 5mg/ml Your baby’s doctor will decide exactly of treatment with other medicines given at the same Please read all of this leaflet carefully how long your newborn must continue Reporting of side effects time. A premature baby may need many before your baby is given this therapy with Caffeine Citrate 10mg/ml If your newborn gets any side effects, medicines, and any problems with caffeine medicine. Oral Solution. If your baby has 5 to 7 days talk to your baby’s doctor. This includes are likely to be minor, but tell the doctor • Keep this leaflet. You may need to read without apnoea attacks, the doctor will any possible side effects not listed in this about any other medication they may it again. stop treatment. leaflet. You can also report side effects D04359 not know about, particularly any other • If you have further questions, please The doctor may decide to check the directly via Yellow Card Scheme. medicine (for example theophylline) given ask the hospital doctor who is looking levels of caffeine in a blood sample Website: www.mhra.gov.uk/yellowcard to your baby to help it breathe. after your baby. as a precaution, or if your baby is not or search for MHRA Yellow Card in the If your newborn gets any side effects, Medications containing phenobarbitone responding to treatment as expected. -

Nevada Medicaid Formulary
Nevada Medicaid-Approved Preferred Drug List Effective August 15, 2021 Legend In each class, drugs are listed alphabetically by either brand name or generic name. Brand name drug: Uppercase in bold type Generic drug: Lowercase in plain type AL: Age Limit Restrictions DO: Dose Optimization Program GR: Gender Restriction OTC: Over the counter medication available with a prescription. (Prescribers please indicate OTC on the prescription) PA: Prior authorization is required. Prior authorization is the process of obtaining approval of benefits before certain prescriptions are filled. QL: Quantity limits; certain prescription medications have specific quantity limits per prescription or per month. SP: Specialty Pharmacy ST: Step therapy is required. You may need to use one medication before benefits for the use of another medication can be authorized. Drug Name Reference Notes *ADHD/ANTI-NARCOLEPSY/ANTI- OBESITY/ANOREXIANTS* *ADHD AGENT - SELECTIVE ALPHA ADRENERGIC AGONISTS*** clonidine hcl er oral tablet extended release Kapvay AL; QL 12 hour *ADHD AGENT - SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR*** atomoxetine hcl oral capsule Strattera DO; AL; QL *AMPHETAMINE MIXTURES*** amphetamine-dextroamphet er oral capsule extended release 24 hour 10 mg, 15 mg, 5 Adderall XR DO; AL; QL mg amphetamine-dextroamphet er oral capsule extended release 24 hour 20 mg, 25 mg, 30 Adderall XR AL; QL mg amphetamine-dextroamphetamine oral Adderall DO; AL; QL tablet 10 mg, 12.5 mg, 15 mg, 5 mg, 7.5 mg amphetamine-dextroamphetamine oral Adderall AL tablet 20 mg, -

Clenbuterol Elisa Kit Instructions Product #101219 & 101216 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology CLENBUTEROL ELISA KIT INSTRUCTIONS PRODUCT #101219 & 101216 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Clenbuterol and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Clenbuterol ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug- enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution. -

12.2% 122,000 135M Top 1% 154 4,800
View metadata, citation and similar papers at core.ac.uk brought to you by CORE We are IntechOpen, provided by IntechOpen the world’s leading publisher of Open Access books Built by scientists, for scientists 4,800 122,000 135M Open access books available International authors and editors Downloads Our authors are among the 154 TOP 1% 12.2% Countries delivered to most cited scientists Contributors from top 500 universities Selection of our books indexed in the Book Citation Index in Web of Science™ Core Collection (BKCI) Interested in publishing with us? Contact [email protected] Numbers displayed above are based on latest data collected. For more information visit www.intechopen.com Chapter A Detail Chemistry of Coffee and Its Analysis Hemraj Sharma Abstract This review article highlights the detailed chemistry of coffee including its components; chemical constituents like carbohydrates, proteins, lipids, and caf- feine; aromatic principles; oil and waxes; and minerals and acids. The high extent of caffeine can be found in the coffee plants; hence, in the second part of the study, various analytical methods are designed for the proper identification, separation, optimization, purification, and determination of caffeine present in coffee, tea, and marketed coffee. These analytical methods are appropriated for the separation and quantification of caffeine. The various analytical methods include spectroscopy methods like UV, IR, and NMR spectroscopy; chromatographic methods like paper, TLC, column, HPLC, and gas chromatography; and hyphenated techniques like LC–MS, GC–MS, and GC–MS/MS. This article compares and contrasts the amount of caffeine by various analytical methods. Keywords: caffeine, spectrophotometer, chromatography, hyphenated techniques, electrochemical methods 1.