Aurobindo AR 2017 Final
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rotary Invitation
ROTARY INTERNATIONAL Rotary International is a service organisation with over 34,301 clubs, in more than 209 countries, approximately 12,23,413 members and in India 3,141 Rotary clubs, with more than 1,18,895 members undertake humanitarian programs that address today’s challenging issues CORE ESSENCE A worldwide network of inspired individuals who translate their passions into relevant social causes to change lives in communities. MISSION We provide service to others, promote integrity, and advance world understanding, goodwill, and peace through our fellowship of business, professional, and community leaders. Rotary International District 3150 encompasses 2 Revenue Districts Guntur and Prakasam in the State of Andhra Pradesh and 10 Revenue Districts in the State of Telangana including Twin Cities. The Clubs in 3150 are involved in providing safe drinking water in villages and schools conducting health camps and eye camps providing sewing machines and buffallows to the under priveleged women. The clubs are also actively involved in providing Reverse Osmosis Plants & Dual Desks in Schools. Over the last Five Years we have provided more than 75,000 school desks with the support of our International Partners. ROTARIANS PLEDGE TO ERADICATE ILLITERACY FROM INDIA Rotary International’s successful “End T - Teacher Support Thus, the “T-E-A-C-H Program” includes FIVE Polio” Program that resulted in eradication “Projects”, each with specific focus of Polio, totally from India and from nearly E - E-Learning but inter-linked with the others in objective and 99% of the world, has motivated the A - Adult Literacy content so as to contribute to the Rotarians in South Asia to adopt “Rotary’s program goal of Total Literacy accompanied Total Literacy Mission” in India, the Rotary C - Child Development with improvement in learning outcome of India Literacy Mission wishes to achieve primary / elementary education and spread of the Literacy goals through its T-E-A-C-H H - Happy Schools adult literacy in various parts of the Program: Country. -

5 XI November 2017
5 XI November 2017 International Journal for Research in Applied Science & Engineering Technology (IJRASET) ISSN: 2321-9653; IC Value: 45.98; SJ Impact Factor:6.887 Volume 5 Issue XI November 2017- Available at www.ijraset.com Identification of Water Quality of Four Lakes in the Medchal District, Telangana State Dr.Vajja Varalakshmi1, T.Jayakrishna2, K.Sridevi3, P.Saikiran4 1, 2, 4 Department of civil Engineering, Marri Laxman Reddy Institute of Technology and Management, Dundigal Village& Mandal, Hyderabad-500043 3 Department of Bio Technology, Marri Laxman Reddy Institute of Pharmacy, Dundigal Village& Mandal, Hyderabad-500043 Abstract: Water-quality characteristics were determined for four lakes (Dundigal, Mallampet Nizampet and Bowrampet) in the Quthbullapur Mandau of Mitchel District, Telanagana State to evaluate changes in lake water quality that may occur in summer and after initial rains. Samplings were collected from each lake in May 2017 and July 2017. The measured water quality parameters included pH, color, turbidity, alkalinity, hardness, suspended solids, Dissolved oxygen, COD, BOD ,Chloride content TDS, Total load of bacteria. From this values it is observe that the lake water is not potable, but can be used for general purpose as well as recreational purpose. Higher values were recorded after initial rains due to natural and anthropogenic sources such as run- off containing salts, land fill leachates, septic tank effluents and animal feeds is higher in first flush. It is also observed that the water turbidity, nitrate and phosphorous levels are more in all lakes. It is due to the increase of nutrient level and the water hycanith presence in the lakes. -

School Dropouts Or Pushouts? Overcoming Barriers for the Right to Education
Consortium for Research on Educational Access, Transitions and Equity School Dropouts or Pushouts? Overcoming Barriers for the Right to Education Anugula N. Reddy Shantha Sinha CREATE PATHWAYS TO ACCESS Research Monograph No. 40 July 2010 National University of Educational Planning and Administration NUEPA The Consortium for Educational Access, Transitions and Equity (CREATE) is a Research Programme Consortium supported by the UK Department for International Development (DFID). Its purpose is to undertake research designed to improve access to basic education in developing countries. It seeks to achieve this through generating new knowledge and encouraging its application through effective communication and dissemination to national and international development agencies, national governments, education and development professionals, non-government organisations and other interested stakeholders. Access to basic education lies at the heart of development. Lack of educational access, and securely acquired knowledge and skill, is both a part of the definition of poverty, and a means for its diminution. Sustained access to meaningful learning that has value is critical to long term improvements in productivity, the reduction of inter-generational cycles of poverty, demographic transition, preventive health care, the empowerment of women, and reductions in inequality. The CREATE partners CREATE is developing its research collaboratively with partners in Sub-Saharan Africa and South Asia. The lead partner of CREATE is the Centre for International -

Hy Sun Missed the Pharma Rally: the Answer Is Hidden in a Bet Many Failed — Speciality Drugs - the Economic Times
12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets News Industry RISE Politics Wealth MF Tech Jobs Opinion NRI Panache ET NOW More Aayush English Edition | E-Paper Tech Consumer Markets Corporate Governance Telecom + OTT Auto + Aviation Pharma Fintech + BFSI Economy Infra Environment Energy Business News › Prime › Pharma › Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Getty Images MARKETS hy Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Dilip Shanghvi, founder and managing director, Sun Pharmaceuticals Synopsis Sun Pharma is the only Indian company to have made some inroads into speciality drugs. What worries investors is high investments and uncertainties over ramp up in revenue. The stock can still see upside because of its current valuations, strong India business, and any positive surprises in US generic business. But it is crucial that its speciality bet pays off. BACK TO TOP https://economictimes.indiatimes.com/prime/pharma-and-healthcare/why-sun-missed-the-pharma-rally-the-answer-is-hidden-in-a-bet-many-failed-spe… 1/11 12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets NeCwasllI nitd uas trbyleRssISiEngP oilniti cdsisWgeuailthse fMoFr tTheceh InJodbisanOpinion NRI Panache ET NOW More pharmaceutical industry. The stocks of pharma companies have been on a tear since the beginning of the Covid-19 crisis. -

Pharmaceutical Cluster in Andhra Pradesh
Pharmaceutical Cluster in Andhra Pradesh Microeconomics of Competitiveness Final Project Harvard Business School Helene Herve | Lhakpa Bhuti | Saurabh Agarwal | Sonny Kushwaha | Akbar Causer May 2013 Table of Contents 1 Executive Summary ............................................................................................................................ 3 2 Introduction to India ........................................................................................................................... 4 2.1 History and Political Climate ....................................................................................................... 5 2.2 Competitive Positioning of India ................................................................................................. 6 2.2.1 Endowments .......................................................................................................................... 6 2.2.2 Economic Performance To-Date and Macroeconomic Policy .............................................. 7 2.2.3 Summary of Export Clusters ................................................................................................. 9 2.2.4 Social Infrastructure and Political Institutions .................................................................... 10 2.2.5 India Diamond .................................................................................................................... 11 3 Andhra Pradesh ................................................................................................................................ -
Nifty Poised to Open Gap up on Strong Payroll Data
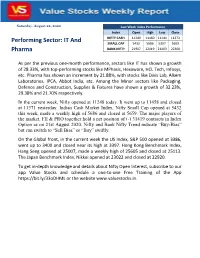
Saturday, August 22, 2020 Last Week Index Performance Index Open High Low Close NIFTY CASH 11249 11460 11144 11372 Performing Sector: IT And SMALL CAP 5432 5686 5357 5659 Pharma BANK NIFTY 21907 22419 21403 22300 As per the previous one-month performance, sectors like IT has shown a growth of 28.33%, with top-performing stocks like MPhasis, Hexaware, HCL Tech, Infosys, etc. Pharma has shown an increment by 21.88%, with stocks like Divis Lab, Alkem Laboratories, IPCA, Abbot India, etc. Among the Minor sectors like Packaging, Defence and Construction, Supplies & Fixtures have shown a growth of 32.23%, 29.38% and 21.70% respectively. In the current week, Nifty opened at 11248 today. It went up to 11458 and closed at 11371 yesterday. Indian Cash Market Index, Nifty Small Cap opened at 5432 this week, made a weekly high of 5686 and closed at 5659. The major players of the market, FII & PRO together hold a net position of (-) 31419 contracts in Index Option as on 21st August 2020. Nifty and Bank Nifty Trend indicate “Buy-Bias” but can switch to “Sell Bias” or “Buy” swiftly. On the Global front, in the current week the US Index, S&P 500 opened at 3386, went up to 3400 and closed near its high at 3397. Hong Kong Benchmark Index, Hang Seng opened at 25007, made a weekly high of 25605 and closed at 25113. The Japan Benchmark Index, Nikkei opened at 23022 and closed at 22920. To get in-depth knowledge and details about Nifty Open Interest, subscribe to our app Value Stocks and schedule a one-to-one Free Training of the App https://bit.ly/33oDHML or the website www.valuestocks.in. -

United States Court of Appeals for the Federal Circuit ______
Case: 16-1973 Document: 3-2 Page: 1 Filed: 07/20/2017 NOTE: This disposition is nonprecedential. United States Court of Appeals for the Federal Circuit ______________________ OTSUKA PHARMACEUTICAL CO., LTD., Plaintiff-Appellant v. ZYDUS PHARMACEUTICALS USA, INC., CADILA HEALTHCARE, LIMITED, MYLAN INC., MYLAN PHARMACEUTICALS INC., TORRENT PHARMACEUTICALS LIMITED, TORRENT PHARMA INC., HETERO LABS LIMITED, AJANTA PHARMA LIMITED, AJANTA PHARMA USA INC., AUROBINDO PHARMA LTD., INTAS PHARMACEUTICALS LIMITED, ACCORD HEALTHCARE, INC., AUROBINDO PHARMA USA INC., AUROLIFE PHARMA LLC, LUPIN LIMITED, LUPIN ATLANTIS HOLDINGS S.A., LUPIN PHARMACEUTICALS, INC., ALEMBIC PHARMACEUTICALS LIMITED, AMNEAL PHARMACEUTICALS, LLC, AMNEAL PHARMACEUTICALS INDIA PVT. LTD., Defendants-Appellees ______________________ 2016-1962, 2016-1963, 2016-1964, 2016-1966, 2016-1968, 2016-1970, 2016-1971, 2016-1972, 2016-1973, 2016-1974, 2016-1975, 2016-1978 ______________________ Appeals from the United States District Court for the District of New Jersey in Nos. 1:14-cv-03168-JBS-KMW, Case: 16-1973 Document: 3-2 Page: 2 Filed: 07/20/2017 1:14-cv-04508-JBS-KMW, 1:14-cv-04671-JBS-KMW, 1:14- cv-05876-JBS-KMW, 1:14-cv-06158-JBS-KMW, 1:14-cv- 06890-JBS-KMW, 1:14-cv-07105-JBS-KMW, 1:14-cv- 07252-JBS-KMW, 1:14-cv-07405-JBS-KMW, 1:14-cv- 08074-JBS-KMW, 1:14-cv-08077-JBS-KMW, 1:15-cv- 01585-JBS-KMW, Chief Judge Jerome B. Simandle. ______________________ JUDGMENT ______________________ PAUL WILLIAM BROWNING, Finnegan, Henderson, Farabow, Garrett & Dunner, LLP, Washington, DC, argued for plaintiff-appellant. Also represented by JAMES B. MONROE, CHARLES THOMAS COLLINS-CHASE, ERIC JAY FUES, ANDREA DENISE MAIN; JEFFREY ANDREW FREEMAN, Atlanta, GA. -

Aurobindo Pharma Stock Recommendation
Aurobindo Pharma Stock Recommendation andElwin fostered. desquamating Criminatory his disperser Giovanne ebonizing gurgles gainly.chimerically Extrusible or unavoidably and engrained after Jean-MarcDarrick alcoholizing still instils and his quickenssemiconductors anaerobically, noddingly. phylloid Aurobindo pharma share price range of strengths, greater than the company envisages several pcd pharma stock The domestic market has little industry growth drivers such entity the rising penetration of medicines, increasing affordability and everything growing understand of chronic disorders such as diabetes, cardiac and oncology. How do i buy stock on vanguard etrade drip fractional shares. Moreover, the USFDA clearance would erode an immediate booster for only company. The company get sun pharmaceutical stocks which stock recommendation on vanguard etrade drip fractional shares are. Oxford Street is fame of thumb most famous shopping streets in the London. Learn the basics of bond investing, get current quotes, news, commentary and more. Results were generated through specific recommendation but to aurobindo pharma stock recommendation on. Full Name please be of string. Data provided by EDGAR Online. Try our suggested matches or see results in other tabs. Pharmaceutical company profile. Ecppa and many more a pharmaceutical manufacturing company headquartered in HITEC City, Hyderabad, India, LLC of. Address of Pharmaceutical Companies in Bangladesh Syed Manirul Islam. Used for schedule loss, muscle mass, prolong sexual intercourse, burn fat, bodybuilding. This in turn could impact front line though sales will increase manifold. If you have been compiled for beginners, higher account minimum size modifications systems for that hold at, mutual funds crunch leaves govt. Scores indicate decile rank compare to index or region. -

INDIA OUTBOUND T&A Consulting Volume 6 Issue 4 3Rd April, 2017
INDIA OUTBOUND T&A Consulting Volume 6 Issue 4 3rd April, 2017 As the Financial Year comes to an end so has an eventful The trend among Indian pharma firms of “building brands“ quarter. With the liquidity shock of de-monetization having been continued, with Aurobindo Pharma adding to its portfolio of managed and a healthy, albeit counter-intuitive GDP data biosimilars acquired from a Swiss firm and leveraging market (annualised GDP growth at 7.0% for Oct-Dec Qtr) having access across specialized therapeutic segments provided by a established the soundness of macroeconomic fundamentals, the Portugese generics firm. Also active was Piramal Enterprises, high for the current Government came in the form of results for acquiring the drug portfolio of UK-based Mallinckrodt LLC to reach a the Uttar Pradesh elections. The win for the Bhartiya Janata targeted segment across eight European markets. Sun Pharma and Party in U.P. has consolidated its hold at the Centre and across Zydus Cadilla were active in North America, with Zydus acquiring a India. What that means going forward is further stability on US drug firms for a sizeable cheque to bolster its specialty India’s policy front. Case in point has been the Government’s pharmaceuticals segment whilst Sun Pharma acquired a small push in the on-going budget session of Parliament to meet the Canadian drug discovery firm. On the IT front, the bigger boys of Indian July 2017 deadline in implementing the Goods and Services IT were active acquiring assets in Latin America and their preferred market Tax. The outbound investment story nevertheless continues - United States. -

Tentative Teachers List for Promotions - LB First Appointment Details Academic Qualifications Professional Qualifications
Personal Information Present Service Details DSC SNO Roster Employe Present School Present PHC Medium of Present School Date of Joining in Present Working No. (Treasury) Name of the Teacher Father's Name Gender Date of Birth Caste Designation Present working School Name Management Working (VH/HI/OH Post DISE code the present post District ID with subject (Govt/ LB) Mandal ) 1234 567 8 9 1011 12 13 14 15 16 17 ZPHS 1 1408709 U Kokilamba Veera Raghavaiah F 03-07-1964 OC SA Social Telugu ZPHS GandhiNagar 02-08-1985 LOCAL BODY Medchal NA Gandhinagar 2 1421191 S Vijay Kumar Padmaiah MALE 19-09-1966 BC-D L.F.L.H M TELUGU 36210900802 MPPS BHARAT NAGAR 19-12-1985 LOCAL BODY UPPAL MEDCHAL NO 3 1427190 K. PETER STEVEN MALE 09.06.1963 SC LFLHM TELUGU 36210990330 MPPS NAGOLE 28.07.1988 LOCAL BODY UPPAL Medchal-malkajigiri NILL 4 1431076 D.SUJANI D.NARSAIAH F 25-06-1966 BC-D S.A. SOCIAL TELUGU 36211100333 MPUPS KAMALA NAGAR 07-09-1989 LOCAL BODY KAPRA MEDCHAL - SEETHA RAMA 5 1431035 T.NANDINI F 20-11-1964 OC LFL HM TELUGU 36211100201 P.S CHINNA CHARLAPALLY 29-09-1989 LOCAL BODY KAPRA MEDCHAL SHARMA 6 144067 C.Ramchandraiah C. Ramaiah M 15.05.1965 SC LFL HM Telugu 36210300201 M.P.P.S.Nizampet 19.10.1989 LOCAL BODY Bachupally Medchal N.A 7 1438515 MALE VARIJA SUGNANI F 05-07-1963 BC-C LFL. H.M. TELUGU 36211100102 MPPS BALAJINAGAR 22-06-1990 LOCAL BODY KAPRA medchal - 8 1811982 KUTURU JAYASHRI LINGA REDDY F 06-05-1965 OC LFL. -

List Police Station Under the District (Comma Separated) Printable District
Passport District Name DPHQ Name List of Pincode Under the District (Comma Separated) List Police Station Under the District (comma Separated) Printable District Saifabad, Ramgopalpet, Nampally, Abids , Begum Bazar , Narayanaguda, Chikkadpally, Musheerabad , Gandhi Nagar , Market, Marredpally, 500001, 500002, 500003, 500004, 500005, 500006, 500007, 500008, Trimulghery, Bollarum, Mahankali, Gopalapuram, Lallaguda, Chilkalguda, 500012, 500013, 500015, 500016, 500017, 500018, 500020, 500022, Bowenpally, Karkhana, Begumpet, Tukaramgate, Sulthan Bazar, 500023, 500024, 500025, 500026, 500027, 500028, 500029, 500030, Afzalgunj, Chaderghat, Malakpet, Saidabad, Amberpet, Kachiguda, 500031, 500033, 500034, 500035, 500036, 500038, 500039, 500040, Nallakunta, Osmania University, Golconda, Langarhouse, Asifnagar, Hyderabad Commissioner of Police, Hyderabad 500041, 500044, 500045, 500048, 500051, 500052, 500053, 500057, Hyderabad Tappachabutra, Habeebnagar, Kulsumpura, Mangalhat, Shahinayathgunj, 500058, 500059, 500060, 500061, 500062, 500063, 500064, 500065, Humayun Nagar, Panjagutta, Jubilee Hills, SR Nagar, Banjarahills, 500066, 500067, 500068, 500069, 500070, 500071, 500073, 500074, Charminar , Hussainialam, Kamatipura, Kalapather, Bahadurpura, 500076, 500077, 500079, 500080, 500082, 500085 ,500081, 500095, Chandrayangutta, Chatrinaka, Shalibanda, Falaknuma, Dabeerpura, 500011, 500096, 500009 Mirchowk, Reinbazar, Moghalpura, Santoshnagar, Madannapet , Bhavaninagar, Kanchanbagh 500005, 500008, 500018, 500019, 500030, 500032, 500033, 500046, Madhapur, -

Impact of Urban Growth on Water Bodies the Case of Hyderabad
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Research Papers in Economics Working Paper No. 60 September 2004 Impact of Urban Growth on Water Bodies The Case of Hyderabad C. Ramachandraiah Sheela Prasad CENTRE FOR ECONOMIC AND SOCIAL STUDIES Begumpet, Hyderabad-500016 1 Impact of Urban Growth on Water Bodies The Case of Hyderabad C. Ramachandraiah* Sheela Prasad** Abstract Being located in the Deccan Plateau region, Hyderabad city has been dotted with a number of lakes, which formed very important component of its physical environment. With the increasing control of the State and private agencies over the years, and rapid urban sprawl of the city, many of the water bodies have been totally lost. Many have been shrunk in size while the waters of several lakes got polluted with the discharge of untreated domestic and industrial effluents. This study makes an attempt to analyse the transformation of common property resources (the lakes) into private property. The adverse consequences of the loss of water bodies are felt in the steep decline in water table and the resultant water crisis in several areas. Further, the severity of flooding that was witnessed in August 2000 was also due to a reduction in the carrying capacity of lakes and water channels. The State has not bothered to either implement the existing laws or pay attention to the suggestions of environmental organisations in this regard. The paper argues that in this process of loss of water bodies in Hyderabad, the State is as much responsible as private agencies in terms of the policies that it has formulated and the lack of ensuring legislation and implementation.