Groundwater Quality Monitoring 2012-13
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The New Zealand Gazette. 2879
Nov. 6.J THE NEW ZEALAND GAZETTE. 2879 279618 Bankier, Charles Nigel, Farm Hand, care of Mr. Grimes, 313277 Beswick, Whitwell John, Pensioner, Emanuera, 1st Avenue, Ngarua Rural Delivery, Waitoa. Tauranga. 277454 Banks, Sefton Joseph, Farmer, Marere, Tirau. 257465 Betteridge, Francis William Cecil, Dairy-farmer, Ohinepanea 068740 Barbalich, Francis Joseph, Roadman, Kopu, Thames. Rural Delivery, Te Puke, Bay of Plenty. 236675 Barbalich, William Nicholas, Stock-agent and Farm Worker, 296502 Bevan, William, Farmer, P.O. Box 23, Whakatane. Kanaeranga, Thames. 284412 Bigland, Charles Ernest, Farmer, care of Mr. E. Kuttell, 251859 Barber, Charles Cyril, Cheesemaker, New Zealand Co-op. Rural Delivery, Putaruru. Dairy Co., Matamata. 253697 Bigwood, Percy Valentine, Share-milker, No. 1 Rd, Waitoa. 247175 Barbour, Charles Humphrey, Farm Hand, Woodlands, 406896 Bilderbeck, Stanley Roland, Farm Hand, Omokorqa, Opotiki. Tauranga. 430672 Barker, John Lawrence, Factory Hand, care of Hostel, 265471 Billing, Alfred Charles, Surveyor's Chainman, Moanataiari Waharoa. Creek, Thames. 201999 Barlow, Alfred James, Dairy-factory ABBistant, 6th Avenue, 372910 Billing, Howard Lawrence, Theatre Employee, Ruakaka, Tauranga. Te Aroha. 092282 Barnacott, John Marshall, Carpenter, P.O. Box 29, Tauranga. 277681 Billing, Robert John, Factory Hand, care of Cheese Factory; 092281 Barnacott, William Frank, Carpenter, P.O. Box 29, Tauranga. Ngatea. 099125 Barnett, Richard Albert, Farm Hand, Omahu, Thames. 289258 Bird, William Gavin, Clerk, Arapuni. 422747 Barney, Ernest, Farmer, Te Puke. 252336 Bishop, Cecil Arnold, Farmer, Harris Rd, Putaruru. 408041 Barney, Frank Lynal, Boot-repairer, Tauranga. 264555 Bishop, Stephen George, Van.-driver, 39 Stewart St, Whab- 305934 Barrell, Arthur Inwood Mark, Dairy Grader and Tester, tane. Waitoa. 241455 Black, Arthur Gordon, School-teacher, care of the School, 258655 Barrett, James William, Farm Hand, Waihi Rd, Paeroa. -

Council Agenda
A G E N D A COUNCIL MEETING Wednesday, 26 June 2019, 9.00am Council Chambers Council Office William Street Paeroa Membership Mayor J P Tregidga Councillors Cr D A Adams (Deputy Mayor) Cr P D Buckthought Cr C Daley Cr R Harris Cr G R Leonard Cr M McLean Cr P A Milner Cr A Rattray Cr D Smeaton Cr A M Spicer Cr D H Swales Cr J H Thorp Executive Leadership L D Cavers D Peddie A de Laborde P Thom S Fabish Public Distribution Paeroa Office Plains Area Office Waihi Area Office Chief Executive L D Cavers Hauraki District Council, P O Box 17, William Street Paeroa, New Zealand P: 07 862 8609 or 0800 734 834 (within the District) E: [email protected] www.hauraki-dc.govt.nz Ngà Karakia Timatanga (opening) (1) Kia tau te rangimarie May peace be widespread Kia whakapapa pounamu te moana May the sea be like greenstone Hei huarahi ma tatou i te rangi nei A pathway for us all this day Aroha atu, aroha mai Let us show respect for each other Tatou i a tatou katoa For one another Hui e! Taiki e! Bind us all together! (2) Whakataka te hau ki te uru, Get ready for the westerly Whakataka te hau ki te tonga. and be prepared for the southerly. Kia mākinakina ki uta, It will be icy cold inland, Kia mātaratara ki tai. and icy cold on the shore. E hī ake ana te atākura he tio, May the dawn rise red-tipped on ice, he huka, he hauhunga. -

THE NEW ZEALAND GAZETTE. [No
2880 THE NEW ZEALAND GAZETTE. [No. llO 118663 Brocklehurst, Frank; Farm Hand, Waitakaruru. 272237 Cameron, Thomas Murray; Pig-farmer, care of C.. N; Walton, 295426 Brockelsby, Charles Kempe, Post-office, Hinuera. Rural :M:ail Delivery, Whakatane. 294599 Brooke; Charles Lee, Farm Hand, Eastport Rd, Waihou, 426279 Campbell, Donald George, Farmer,. Otakiri, Whakatane. Te Aroha. 251841 Campbell, Gor.don Oliver,.Grocery Assistant, care of Wallace 018942 Brooking, Ronald Ernest, Miner, care of Mrs. Stewart, Supplies, Matamata. · 80 Kenny St, Waihi. 240952 Campbell, Ivan Hill, Painter, 40 Tenth Avenue, Tauranga. 281351 Brooks, Edward Lester, Post-splitter, care of Post-office, 231317 Campbell, James Alexander, Orderman, Arahlwi Rd, Mokai. Mamaku. 097779.Brophy, Daniel James, Farmer, Otakiri, Bay of Plenty. 292963 Campbell, Robert, Kauri Point, Katikati. 295423 Brown, Christopher Oaksford (Jun.), Farm Labourer, care 426635 Campbell, William McKenzie, Salesman, Otahiri Rural · of·Mr. G. Miller, Ngarua, Waitoa. Delivery, Whakatane. 422712 Brown, Ernest Edward, Farmer, Matatoki Rd, :M:atatoki. 276825 Cannell, Robert Lewis, Grocer's Assistant, Katikati. 375971 Brown, Louis James Durieu, Farmer, Tauhei, Morrinsville. 191217 Qannell, Thomas Arthur, Exchange Clerk, Katikati. 236054 Brown, Roland, Driller, 90 Brown St, Thames, 236060 Cardno, Colin Grierson, Cheesemaker, care of New Zealand 236058 Brown; Stanley Edwin, Shop-assistant, care of Mr. S. Ensor, co:op. Dairy Co., Ltd., Hikutaia. Mackay St, Thames. 268932 Carlyle, George Leonard, Share-milker, Okoroire. 283255 Brown Thomas, Dairyman, Waharoa. 423298 Carmichael, Cyril Gordon, Dairy-farmer, Bethlehem Rural 054439 Brown, William, Farmer, Tatuanui. Delivery, Tauranga. 167837 Brown, William Graham, Labourer, care of. Grande Vue ·265032 Carr, James Harry, Butcher, Tanding Rd, Whakatane. Private Hotel, Whakatane. -

Minutes of Ordinary Meeting of Matamata-Piako District Council
Kaunihera | Council Mēneti Wātea | Open Minutes Minutes of a meeting of Matamata-Piako District Council held on Wednesday, 13 May 2020, at 9am. Ngā Mema | Membership Mayor Ash Tanner, JP (Chair) Deputy Mayor Neil Goodger District Councillors Donna Arnold Caitlin Casey Teena Cornes Bruce Dewhurst James Sainsbury Russell Smith Kevin Tappin James Thomas Sue Whiting Adrienne Wilcock Council 13 May 2020 Ngā whakapāha | Apologies Kaimahi i reira | Staff Present Name Title Item No. Don McLeod Chief Executive Officer Michelle Staines-Hawthorne Corporate Strategy Manager Tamara Kingi Committee Secretary and Corporate Administration Officer Sandra Harris Senior Policy Planner Ann-Jorun Hunter Policy Planner Niall Baker Senior Policy Planner Dennis Bellamy Group Manager Community Development Fiona Vessey Group Manager Service Delivery Jennie Cochrane Communication Manager Amy Pollock Procurement Officer 7.1 Bryan Turner Coordinator Operations and Projects 7.7 I reira | In Attendance Time In Time Out Rien van de Weteringh – Representative of Waikato 10.00am 10.20am Regional Council and Keep Te Aroha Beautiful Minutes Page 2 Council 13 May 2020 1 Whakatūwheratanga o te hui | Meeting Opening Mayor Ash Tanner declared the meeting open at 9.02am. 2 Ngā whakapāha | Apologies / Tono whakawātea | Leave of Absence There were no apologies. No leave of absence was requested. 3 Pānui take ohorere | Notification of Urgent Business Pānui tāpiritanga take | Notification of Additional Business Section 46A(7) of the Local Government Official Information and Meetings -

Product Update Autumn 2019
Product Update Autumn 2019 Discover the latest product updates Helensville for the Waikato 1 Situated in the central North Island we’reTown/City famousRoad forState Middle- Thermal Waikato Hamilton i-SITE Information Highway Explorer River Airport Visitor Info Centre earth movie magic, underground wonders, the iconic WaikatoHighway Centre River, legendary surf beaches and that’s just the start… Gravel Cycle Trails Thermal Surf Waterfall Forest Mountain Caves Contact the trade team at Hamilton & WaikatoRoad TourismGeyser toBeach Range AUCKLANDfind out more. We can help with product enquiries, in-depth knowledge of the region, itinerary preparation, regional content and assistance with hosting. Coromandel Peninsula Phone: +64 7 843 0056Clevedon or email [email protected] To Whitianga Miranda Thames Pukekohe WhangamataWhangamataˉ Waiuku POKENOPOˉ KENO To Thames Maramarua 2 MERCER Mangatarata to River TUAKAU Meremere aika W Hampton Downs Hauraki 25 Rail Trail Paeroa PORT WAIKATO Te Kauwhata WaihiWaihiˉ 2 Rangiriri 2 Glen 1 Murray Tahuna 26 Kaimai-Mamaku Forest Park WaikWaikaretuaˉ retu Lake Hakanoa TE AROHA Mt Te Aroha HUNTLY Lake Puketirni 27 26 Waiorongomai Valley Taupiri Tatuanui 2 HukarimataHaˉkarimata 1B Ranges Gordonton Kaimai Ranges Te Akau NGARUAWAHIANGAˉRUAWAˉ HIA MORRINSVILLE Te Awa Ngarua Waingaro River Ride TAURANGA 39 2 Horotiu 27 Wairere Walton Falls Raglan HAMILTON New section of Waharoa Harbour Hauraki Rail Trail 2 Whatawhata RAGLAN Matangi due to open mid 2019 Manu Bay Tamahere 1B 29 23 MATAMATA Te Puke Mt Karioi Raglan Trails CAMBRIDGE Introducing our 29 Ngahinapouri Ruapuke ˉ 27 Beach OhaupoOhaupoˉ Te Awa River Ride Piarere Bridal Veil Falls / 3 Lake new consumer Te Pahu WairengaWaireˉnga Pirongia Forest Park Karapiro 1 Aotea TIRAUTIˉRAU Harbour 5 brand story Mt Pirongia Pirongia Sanctuary TE AWAMUTU Mountain Te Puia Springs Kihikihi Mt Maungatautari Hot Water Beach KAWHIAKAˉ WHIA PUTARURU 33 Pukeatua Arapuni To Rotorua Come with us, share our waka and feel our strength 31 Paraˉraˉwera 5 Tihiroa 3 and passion. -

The New Zealand Gazette. 1689
JUNE 24.] THE NEW ZEALAND GAZETTE. 1689 MILITARY AREA No. 2 (PAEROA)-continued. MILITARY AREA· No.· 2, (PAEROA)-.continued. 555163 Reeve, Herbert Alfred, cheesemaker, Motumaoho, Morrins- 550822 Schollum, Noel Wenzl, far'in-manager, Motumaoho Rural ville. Delivery, Morrinsville. 543751 Reid, Edward, farmer, Waihou. 588710 Scott, Frederick Gordon, farmer, Pekapeka Rd., Kerepehi. 627325 Reid, James Boyd, student, Kereon~ Rd., Morrinsville, 523599 Scott, George MacDonald, farmer, Waharoa. 541816 Reid, Ralph Cuthbert, chemist, Tamihana St., Matamata. 509670 Scott, John Colin, farmer, Turanga-o-Moana, Matamata. 490965 Reid, Ronald Murray Hamilton, schoolmaster, Johnstone 627568 Scott, Robert James, farm hand, Ngarua Rural Delivery, St., Te Aroha. Waitoa. 586226 Reid, William Reginald Clifford, dairy-farmer, Maukoro, 589785 Scott, Walter James, engineer, Pererika St., Rotorua. Patetonga Rural Delivery, Morrinsville. 513384 Scown, Daniel Eric William, railway porter, Upper Richmond 627309 Relf, Clement Alexander, farm hand, Lake Rotoma, Rotorua. St., Thames. · 541702 Rendall, Norman John, sawmiller, Manawahe, Matata. 541263 Searle, Arthur Lewis, fitter, care of Gardiner and Sons, 492358 Rennie, Raymand John, electrical linesman, Waikino. Tawawera Timber Co. Ltd., Te Haroto. 627240 Restall, Ronald Howard, nursery hand, Whangamata, 481582 Sears, Douglas, cream contractor, Waitakaruru. Private Bag, Waihi. 1 628094 Seymour, Robert, farm hand, care of L. M. Fahey, Manga- 584330 Reynolds, Ronald George, farmer, Wharepoa, Thames Line. teparu, Morrinsville. 573271 Reynolds, William Henry Ellis, dairy-farmer, Te Puke. 587284 Sharp, William Campbell, farmer, care of R. Charles, 512929 Richards, Hector Percival, contractor, David St., Morrins- Kereone. ville. • 627807 Sharpe, Gary Wilfred Glenville, garage apprentice, Waharoa 511669 Richards, Reginald Francis, Hotel Imperial, Pollen St., Rd., Matamata. Thames. 532431 Shaw, Donald, labourer, D11vy St., Thames. -

Te Kuiti Piopio Kawhia Raglan Regional
Helensville 1 Town/City Road State Highway Expressway Thermal Explorer Highway Cycle Trails Waikato River REGIONAL MAP Hamilton Airport i-SITE Visitor Information Centre Information Centre Thermal Geyser Surf Beach Water Fall Forest Mountain Range AUCKLAND Coromandel Peninsula Clevedon To Whitianga Miranda Thames Pukekohe Whangamata Waiuku POKENO To Thames Maramarua 2 Mangatarata to River TUAKAU Meremere aika W Hampton Downs Hauraki 25 Rail Trail Paeroa PORT WAIKATO Te Kauwhata Waihi 2 Rangiriri 2 Glen 1 Murray Tahuna 26 Kaimai-Mamaku Mount Forest Park Lake Hakanoa Te Aroha Mt Te Aroha Lake Puketirni HUNTLY TE AROHA 27 26 Waiorongomai Valley Taupiri Tatuanui 2 1B Gordonton Te Akau Te Awa NGARUAWAHIA MORRINSVILLE River Ride Ngarua Waingaro TAURANGA 39 Horotiu 2 27 Walton Wairere Falls Raglan HAMILTON Harbour Waharoa 2 Whatawhata Matangi RAGLAN MATAMATA Manu Bay Tamahere 1B 29 23 Te Puke Mt Karioi Raglan Trails CAMBRIDGE 29 Ngahinapouri Ruapuke 27 Beach Ohaupo Piarere 3 Te Awa Lake Te Pahu Bridal Veil Pirongia Forest Park River Ride Karapiro 1 Aotea Falls TIRAU Harbour 5 Mt Pirongia Pirongia Sanctuary TE AWAMUTU Mountain KAWHIA Kihikihi Mt Maungatautari PUTARURU 33 Pukeatua To Rotorua Parawera Arapuni 5 Kawhia 31 Harbour Tihiroa 3 Te Puia Springs 39 1 ROTORUA Hot Water Beach Waikato Optiki River Trails Taharoa OTOROHANGA WAITOMO CAVES Marokopa Falls 3 TOKOROA To Rotorua Waimahora 1 5 Marokopa TE KUITIKUITI 32 30 Mangakino Rangitoto 3 Pureora Forest Park Whakamaru to River Waika PIOPIOPIOPIO 30 4 Pureora Forest Park 32 3 30 To Taumarunui -

The New Zealand Gazette. 2231
SEPT. 15.] THE NEW ZEALAND GAZETTE. 2231 MILITARY AREA No. 2 (PAEROA)-oontimred. MILITARY AREA No. 2 (PAEROA)-oontimJeil. 599521 Brown, Eustace Gordon, dairy-farmer, Okoroire, Tirau. 522809 Clarey, Albert John, mill hand, care of Tunnicliffe, Ponga• · 582189 Brown, Leonard, dairy-farmer, Te Puke. kawa Rural Delivery~ Te Puke. 582209 Brown, Sydney Herbert, battery hand, Dickson's Track, 527164 Clark, Frank, farmer, care of W. C. Cederman, care of P.O., Waikino. Opotiki. 524939 Brown, William Giles, builder and contractor, Karaka Rd., 507048 Clark, Lindsay Raymond Harrison, Ford St., Opotiki. Thames. 630802 Clark, Mervyn Allen Neil, Brady's Estate, Shaftesbury, Te 472839 Browne, Reginald Robert, school-teacher, Belvedere St., Aroha. Te Puke. 495464 Clark, Raymond Owen, platelayer (N.Z.R.), 53 Hunter St., 582226 Brunt. Herbert, clerk, Flora St. Tauranga. 533052 Brunton, Wallace Colin Thomas, dairy-farmer, Wharepoa. 556848 Clark, Robert John Watkins, clerk, Arthur St. 582222 Bryant, James Thomas Godfrey, farm hand, Te Teko, 488428 Clarkin, Frederick Arthur, tablet porter (N.Z.R.), care of Whakatane. N.Z. Railways, Tirau. ~ 629994 Buchanan, Clifford Charles, farm hand, Rural Delivery, 480069 Claxton, William Harry, factory-manager, 33 Hills St., Patatere, Tirau. Thames. 512123 Buchanan, Kenneth George, sawmiller, Robin St., Waihi. 519226 Clayton, Benjamin, carpenter, 4 Sonoma Flats, Rukuatua 585075 Budd, Keith Graem'3ly, farmer, No. 2 Rd., Te Puke. St., Rotorua. 632001 Buffalora, Peter, restaurant proprietor, Pollen St., Thames. 631088 Clayton, Ronald Bertram, farm hand, Waiatahi, Opotiki. 507308 Bullpck, Francis Harold, share-milker, Rural Mail Delivery, 527162 Clements, Robert John, farmer, Waihi. Waghorns Rd., Waharoa. 586373 Clemson, Charles Rowland William Hall, bank officer, 592028 Bunn, Francis Harold, share-milker, Otakiri. -
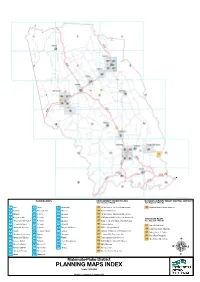
PLANNING MAPS INDEX Scale 1:230,000
PLANNING MAPS DEVELOPMENT CONCEPT PLANS MATAMATA AIRPORT HEIGHT CONTROL SURFACES (See Schedule 5 Part B) (See Appendix 6 Part B) 1 Waiti 16 Piarere 30 Morrinsville 43 NZ Mushrooms Ltd-Snell St Morrinsville 55 Matamata Height Control Surfaces 2 Kopuatai 17 Hinuera-Te Poi 31 Waharoa 44 Fonterra Morrinsville 3 Mangaiti 18 Selwyn 32 Matamata 45 NZ Mushrooms-Taukoro Rd Morrinsville 4 Hangawera Hills 19 Te Aroha 33 Matamata 46 ICHEM Ltd & BOP Fertiliser Ltd Morrinsville STRUCTURE PLANS 5 Mangateparu-Springdale 20 Te Aroha 34 Matamata 47 Tatua co-Operative Diary Co Ltd Tatuanui (See Appendix 9 Part B) 6 Te Aroha Environs 21 Te Aroha 34 Matamata 48 Fonterra Waitoa 57 Banks Rd Matamata 7 Morrinsville Environs 22 Te Aroha 36 Douglas Rd-Okauia 49 Wallace Corporation Ltd 58 Eldonwood South Matamata 8 Ngarua 22a Te Aroha Central 37 Tahuna 50 Inghams Enterprises (New Zealand) Ltd 60 Stirling Street Te Aroha 9 Manawaru-Shaftesbury 23 Waihou 38 Manawaru 51 Te Aroha Skin Processors Ltd 61 Tower Road Matamata 10 Motumaoho-Tahuroa 24 Waitoa 39 Walton 52 Totara Springs Christian Centre 62 Horrell Road Morrinsville 11 Kereone-Walton 25 Tatuanui 40 Tower Rd-Matamata 53 IB & JB Diprose Barton Rd Okauia 12 Wardville-Gordon 26 Morrinsville 41 Te Poi 54 DL & JL Swaps 13 Richmond Downs 27 Morrinsville 42 Hinuera 56 Richmond Ltd 14 Matamata Environs 28 Morrinsville 59 Greenlea Premier Meats Ltd 15 Okauia 29 Morrinsville Matamata-Piako District PLANNING MAPS INDEX Scale 1:230,000 Version 2 -- Operative 21 October 2015 Matamata-Piako District Plan Legend INDUSTRIAL -

6 June 2017 Time: 10:00Am Venue: Council Chambers 35 Kenrick Street TE AROHA
Te Manawhenua Forum Mo Matamata-Piako Open Agenda Notice is hereby given that an ordinary meeting of the Te Manawhenua Forum Mo Matamata- Piako will be held on: Date: Tuesday 6 June 2017 Time: 10:00am Venue: Council Chambers 35 Kenrick Street TE AROHA Membership Mayor Jan Barnes, JP Cr James Thomas, JP Mrs Jill Taylor Mrs Te Ao Marama Maaka Ms Shelley Turner Mr Weka Pene Mrs Kathy Ngamane Mr Thomas Smith Mr Wati Ngamane Mr Michael Baker Mr Phillip Samuels Ms Glenice Wigg Ms Dianna Vaimoso Mr Gary Thompson Phone: 07-884-0060 Address: PO Box 266, Te Aroha 3342 Email: [email protected] Website: www.mpdc.govt.nz Te Manawhenua Forum Mo Matamata-Piako 6 June 2017 ITEM TABLE OF CONTENTS PAGE Procedural 1 Meeting Opening 5 2 Karakia 5 3 Present 5 4 Apologies 5 5 Notification of Urgent Business 5 6 Confirmation of minutes 5 Officer Reports 7 Executive 7.1 Ngati Rāhiri Tumutumu and Ngati Hauā representation 7 7.2 Te Manawhenua Forum Satisfaction Survey 9 7.3 Representation Review 2018 11 7.4 Heads of Agreement Review 25 7.5 Long Term Plan 2018-28 - Project Update 33 7.6 Eastern Waikato Joint Waste Management and Minimisation Plan Update 37 7.7 Easter Trading Policy, Bylaw Reviews, Fees and Charges 41 7.8 Statistical Standard for Geographic Areas 43 7.9 Waitangi Day 2018 47 8 Environmental 8.1 District Plan Update 49 9 Service Delivery 9.1 Waikato Plan - Minutes of hearing 51 9.2 Annual Customer Survey 2017 57 9.3 Matamata-Piako Civic and Memorial Centre 61 10 Urgent Additional Business Page 3 Te Manawhenua Forum Mo Matamata-Piako 6 June 2017 1 Meeting Opening 2 Karakia 3 Present 4 Apologies At the close of the agenda no apologies had been received. -
Official Regional Visitor Guide 2021
OFFICIAL REGIONAL VISITOR GUIDE 2021 HAMILTON • NORTH WAIKATO RAGLAN • MORRINSVILLE TE AROHA • MATAMATA CAMBRIDGE • TE AWAMUTU WAITOMO • SOUTH WAIKATO Helensville 1 Town/City Road State Thermal Waikato Hamilton i-SITE Information Highway Explorer River Airport Visitor Info Centre Highway Centre Gravel Cycle Trails Thermal Surf Waterfall Forest Mountain Caves Road Geyser Beach Range AUCKLAND Coromandel Peninsula Clevedon To Whitianga Miranda Thames Pukekohe Whangamataˉ Waiuku POˉ KENO To Thames Maramarua 2 MERCER Mangatarata to River a TUAKAU Meremere aik W 25 Hampton Downs Drive times - from Hamilton: Paeroa PORT WAIKATO Te Kauwhata Waihiˉ Auckland ................. 1 hr 45 mins 2 Rotorua ................... 1 hr 20 mins Rangiriri Taupō ...................... 1 hr 50 mins 2 Glen 1 Coromandel ............. 2 hr 20 mins Murray Tahuna 26 Kaimai-Mamaku Forest Park Tauranga ................. 1 hr 30 mins Waikaˉ retu Ruapehu .................. 3 hr 05 mins Lake Hakanoa TE AROHA Mt Te Aroha Hawke’s Bay ........... 3 hr 10 mins HUNTLY Tairāwhiti-Gisborne .. 4 hr 45 mins Lake Puketirni 27 26 Waiorongomai Valley Taupiri Hauraki Tatuanui Rail Trail 2 Haˉkarimata 1B Ranges Gordonton Kaimai Ranges Te Akau NGAˉRUAWAˉ HIA MORRINSVILLE Te Awa Ngarua Waingaro River Ride TAURANGA 39 2 Horotiu 27 Wairere Walton Falls Raglan HAMILTON Harbour Waharoa 2 RAGLAN Whatawhata Matangi Manu Bay Tamahere 1B 29 23 MATAMATA Te Puke Mt Karioi Raglan Trails CAMBRIDGE 29 Ngahinapouri Ruapuke ˉ 27 Beach Ohaupoˉ Te Awa River Ride Piarere Bridal Veil Falls / 3 Lake Te Pahu -

PLAN CHANGE 48 Appendix C Proposed Changes to Schedule 3
Page | 40 PLAN CHANGE 48 Appendix C Proposed Changes to Schedule 3 Page | 41 Schedule 3: Outstanding or significant natural features and trees and other protected items See Appendix 1 for evaluation criteria Reg no Location Common names Map no Legal description 1 Aitken Homestead Gingko, Sweet Chestnut, Macrocarpa, Radiata Pine, Silver 8 Part Lot 2 DP 13021 Maungakawa Road Birch, Eucalyptus, Phoenix Palm, Oaks, Silver Fir, Kereone Liquidamber and Redwoods 2 Near Aitken Homestead Cork Oak 8 Lot 1 DPS 24698 Maungakawa Road Kereone 3 Both banks Waitoa River west of Ngarua Lowland Bush 8 Pakarau Pa Block A2 and Part SW 6 Block VIII Memorial Hall Maungakawa SD State Highway 27 Ngarua 4 North of Walton Road at junction with State Kahikatea Lowland Forest 11 Section 23 block VIII, Wairere SD Highway 27 5 State Highway 27/Wairere Road Stand of Kahikatea 11 Part Section 1 & Matamata North, Part 2B1 A2 and 3A block VIII, Wairere SD and Part Lot 2 DPS 12032 6 Walton School Red Oaks 39 Part DP 915A and All DPS 3403, DP 8523, DP 14538 Walton 7 Walton Village Copper Beech 11 Part Lot 2 DP 27651 Walton 7/1 Walton Village Kauri 39 Part Lot 2 DP 27651 Walton 8 Walton Village Sycamore 11 Part Lot 1 DP 7329 Walton 9 Wardville School Tanekaha, Japanese Cedar, Sycamore, Holly, Totara (3), 12 Part Lot 9 DP 2838, and Part Lot 9 DP 4223 Wardville Liquid Amber, Memorial Ginkgo, Kauri 10 Walker/Mills Streets Kahikatea Bush 31 Part Lots 1 and 2 DP 13299, Part 9A DP 850 Lots 1 and 2 Waharoa DPS 26844, Section 77 block XIII Wairere SD and Lot 2 LTS 39444 11 Banks