Sir William Ramsay Discovered Neon, Krypton, and Xenon and Received Nobel Prize in 1904
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
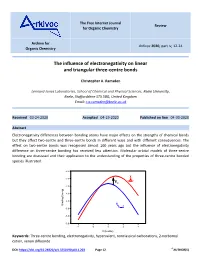
The Influence of Electronegativity on Linear and Triangular Three-Centre Bonds
The Free Internet Journal Review for Organic Chemistry Archive for Arkivoc 2020, part iv, 12-24 Organic Chemistry The influence of electronegativity on linear and triangular three-centre bonds Christopher A. Ramsden Lennard-Jones Laboratories, School of Chemical and Physical Sciences, Keele University, Keele, Staffordshire ST5 5BG, United Kingdom Email: [email protected] Received 03-24-2020 Accepted 04-19-2020 Published on line 04-30-2020 Abstract Electronegativity differences between bonding atoms have major effects on the strengths of chemical bonds but they affect two-centre and three-centre bonds in different ways and with different consequences. The effect on two-centre bonds was recognised almost 100 years ago but the influence of electronegativity difference on three-centre bonding has received less attention. Molecular orbital models of three-centre bonding are discussed and their application to the understanding of the properties of three-centre bonded species illustrated. -2.5 X -3.0 + Y Y Ea -3.5 -4.0 -4.5 X Binding Energy Binding + -5.0 Y Y -5.5 -6.0 -4 -2 0 2 4 h (b units) Keywords: Three-centre bonding, electronegativity, hypervalent, nonclassical carbocations, 2-norbornyl cation, xenon difluoride DOI: https://doi.org/10.24820/ark.5550190.p011.203 Page 12 ©AUTHOR(S) Arkivoc 2020, iv, 12-24 Ramsden, C. A. Table of Contents 1. Introduction 2. Linear Three-Centre Bonds (Hypervalent Bonds) (X-Y-X) X 3. Triangular Three-Centre Bonds (Y Y) 4. The Localised Bond Model of Two- and Three-Centre Bonds 5. Conclusions References -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Midterm Examination #3, December 11, 2015 1. (10 Point
NAME: NITROMETHANE CHEMISTRY 443, Fall, 2015(15F) Section Number: 10 Midterm Examination #3, December 11, 2015 Answer each question in the space provided; use back of page if extra space is needed. Answer questions so the grader can READILY understand your work; only work on the exam sheet will be considered. Write answers, where appropriate, with reasonable numbers of significant figures. You may use only the "Student Handbook," a calculator, and a straight edge. 1. (10 points) Argon is a noble gas. For all practical purposes it can be considered an ideal gas. DO NOT WRITE Calculate the change in molar entropy of argon when it is subjected to a process in which the molar IN THIS SPACE volume is tripled and the temperature is simultaneously changed from 300 K to 400 K. 1,2 _______/25 This is a straightforward application of thermodynamics: 3,4 _______/25 = = + = + 2 2 2 2 5 _______/20 Identifying ∆the derivative� and� doing1 � the� integrals � 1give� � �1 � � �1 3 3 400 6,7 _______/20 = + = 8.3144349 + 8.3144349 2 2 1 2 300 8 _______/10 where the∆ heat capacity � 1� at constant � 1volume� of a monatomic �ideal� gas is� . � � � 3 ============= = 9.13434 + 3.58786 = 12.722202 9 _______/5 ∆ (Extra credit) ============= TOTAL PTS 2. (15 points) Benzene ( • = 96.4 ) and toluene ( • = 28.9 ) form a nearly ideal solution over a wide range. For purposes of this question, you may assume that a solution of the two is ideal. (a) What is the total vapor pressure above a solution containing 5.00 moles of benzene and 3.25 moles of toluene? 5.00 3.25 = • + • = (96.4 ) + (28.9 ) 5.00 + 3.25 5.00 + 3.25 = 58.4 + 11.4 = 69.8 (b) What is mole fraction of benzene in the vapor above this solution? . -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

The Oganesson Odyssey Kit Chapman Explores the Voyage to the Discovery of Element 118, the Pioneer Chemist It Is Named After, and False Claims Made Along the Way
in your element The oganesson odyssey Kit Chapman explores the voyage to the discovery of element 118, the pioneer chemist it is named after, and false claims made along the way. aving an element named after you Ninov had been dismissed from Berkeley for is incredibly rare. In fact, to be scientific misconduct in May5, and had filed Hhonoured in this manner during a grievance procedure6. your lifetime has only happened to Today, the discovery of the last element two scientists — Glenn Seaborg and of the periodic table as we know it is Yuri Oganessian. Yet, on meeting Oganessian undisputed, but its structure and properties it seems fitting. A colleague of his once remain a mystery. No chemistry has been told me that when he first arrived in the performed on this radioactive giant: 294Og halls of Oganessian’s programme at the has a half-life of less than a millisecond Joint Institute for Nuclear Research (JINR) before it succumbs to α -decay. in Dubna, Russia, it was unlike anything Theoretical models however suggest it he’d ever experienced. Forget the 2,000 ton may not conform to the periodic trends. As magnets, the beam lines and the brand new a noble gas, you would expect oganesson cyclotron being installed designed to hunt to have closed valence shells, ending with for elements 119 and 120, the difference a filled 7s27p6 configuration. But in 2017, a was Oganessian: “When you come to work US–New Zealand collaboration predicted for Yuri, it’s not like a lab,” he explained. that isn’t the case7. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

The Oxidizing Behavior of Some Platinum Metal Fluorides
Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory Title THE OXIDIZING BEHAVIOR OF SOME PLATINUM METAL FLUORIDES Permalink https://escholarship.org/uc/item/46b5r4v5 Author Graham, Lionell Publication Date 1978-10-01 eScholarship.org Powered by the California Digital Library University of California LBL-8088.c,y '': ·,~, . ' '~~.. .· J!' ... THE OXIDIZING BEHAV!OROF SOME PLATINUM METAL FLUORIDES . Li onell Graham. · (Ph~ D. Thesis ) IIi !l1/ "~ · r-,> ·1cr7o October 1978 ~t .. ~, ~L l ... ;,() · .. t .. J r.~~ r: !\ ~;~, ·l .• ~~:J"~~ t~J ;,~.)(·_ .. {·~ tJ 1v~ ;;:: r~- J··r·r,;~ Ei:~-:·~,:~~ <'11 ().i\{ Prep~red for the .. U. S. Department of Energy. under Contract W~7405~ENG-48 TWO-WEEK LOAN COPY This is a Library Circulating Copy which may be borrowed for two weeks. .. ' ·t•·! •. ·. For a personal retention copy, call Tech. Info. Diu is ion, Ext. 6782 .. LEGAL NOTICE -___,..-----~__, This report was prepared as an account of work sponsored by the United States Government Neither the United States nor the Depart ment of Energy, nor any of their employees, nor any of their con tractors, subcontractors, or their employees, makes any warraf')ty, express or implied,orassumes any legal liabii bility for the accuracy, completeness or usefulness of nformation, appa- ratus, product. ·· · · disclosed; or rep ·. ts that its use would. not infringe p. · · owned rights ... -i- THE OXIDIZING BEHAVIOR OF SOME PLATINUM METAL FLUORIDES Contents Abstract . v I. General Introduction 1 II. General Apparatus and Handling Techniques 2 A. Apparatus . 2 1. General 2 2. The Cryogenic Technology, Inc., Model 21 Cryo-Cooler .. 3 3. Reactor for Gas-Gas Reaction Products 5 B. -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

Problems for Chapter 17
Molecular Modeling Problems Chapter 17 1. Argon Compounds? One of the major advantages of calculation over experiment is that “reality does not get in the way”. It is no harder to investigate the properties of labile compounds that may be difficult to isolate and characterize experimentally than it is to investigate those of stable compounds. In fact, it is possible to say whether experimental characterization can ever be achieved, that is, if the molecule of interest is actually an energy minimum. Noble gas compounds illustrate this. While xenon compounds are now numerous and a few compounds of krypton have now been reported, no argon compounds have been isolated or characterized. Do argon analogues of known xenon and krypton compounds actually exist (in the sense that they represent energy minima)? If so, do they exhibit similar geometries and charge distribution as their analogues? Obtain equilibrium geometries for argon difluoride, krypton difluoride and xenon difluoride using the Hartree-Fock 3-21G model. Start from bent structures even though. KrF2 and XeF2 are known to be linear (and ArF2 might very well be assumed to be linear as well). While the geometry optimization is able to move from a non-linear to a linear structure, it cannot do the reverse. Follow each optimization by an infrared spectrum calculation to tell you whether or not the structure is actually an energy minimum. Are KrF2 and XeF2 linear molecules? Is the calculated Kr-F bond distance in reasonable accord with the experimental value of 1.89Ǻ (the bond distance in XeF2 linear is not known)? Is ArF2 an energy minimum? Is it linear? If ArF2 is an energy minimum, is dissociation to Ar and F2 endothermic or exothermic? Are the corresponding dissociations of KrF2 and XeF2 endothermic or exothermic? Obtain electrostatic potential maps for the three compounds and display side by side on screen (and on the same scale). -

Chemistry of the Noble Gases*
CHEMISTRY OF THE NOBLE GASES* By Professor K. K. GREE~woon , :.\I.Sc., sc.D .. r".lU.C. University of N ewca.stle 1tpon Tyne The inert gases, or noble gases as they are elements were unsuccessful, and for over now more appropriately called, are a remark 60 years they epitomized chemical inertness. able group of elements. The lightest, helium, Indeed, their electron configuration, s2p6, was recognized in the gases of the sun before became known as 'the stable octet,' and this it was isolated on ea.rth as its name (i]A.tos) fotmed the basis of the fit·st electronic theory implies. The first inert gas was isolated in of valency in 1916. Despite this, many 1895 by Ramsay and Rayleigh; it was named people felt that it should be possible to induce argon (apy6s, inert) and occurs to the extent the inert gases to form compounds, and many of 0·93% in the earth's atmosphere. The of the early experiments directed to this end other gases were all isolated before the turn have recently been reviewed.l of the century and were named neon (v€ov, There were several reasons why chemists new), krypton (KpVn'TOV, hidden), xenon believed that the inert gases might form ~€vov, stmnger) and radon (radioactive chemical compounds under the correct con emanation). Though they occur much less ditions. For example, the ionization poten abundantly than argon they cannot strictly tial of xenon is actually lower than those of be called rare gases; this can be illustrated hydrogen, nitrogen, oxygen, fl uorine and by calculating the volumes occupied a.t s.t.p. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

The Noble Gases
INTERCHAPTER K The Noble Gases When an electric discharge is passed through a noble gas, light is emitted as electronically excited noble-gas atoms decay to lower energy levels. The tubes contain helium, neon, argon, krypton, and xenon. University Science Books, ©2011. All rights reserved. www.uscibooks.com Title General Chemistry - 4th ed Author McQuarrie/Gallogy Artist George Kelvin Figure # fig. K2 (965) Date 09/02/09 Check if revision Approved K. THE NOBLE GASES K1 2 0 Nitrogen and He Air P Mg(ClO ) NaOH 4 4 2 noble gases 4.002602 1s2 O removal H O removal CO removal 10 0 2 2 2 Ne Figure K.1 A schematic illustration of the removal of O2(g), H2O(g), and CO2(g) from air. First the oxygen is removed by allowing the air to pass over phosphorus, P (s) + 5 O (g) → P O (s). 20.1797 4 2 4 10 2s22p6 The residual air is passed through anhydrous magnesium perchlorate to remove the water vapor, Mg(ClO ) (s) + 6 H O(g) → Mg(ClO ) ∙6 H O(s), and then through sodium hydroxide to remove 18 0 4 2 2 4 2 2 the carbon dioxide, NaOH(s) + CO2(g) → NaHCO3(s). The gas that remains is primarily nitrogen Ar with about 1% noble gases. 39.948 3s23p6 36 0 The Group 18 elements—helium, K-1. The Noble Gases Were Kr neon, argon, krypton, xenon, and Not Discovered until 1893 83.798 radon—are called the noble gases 2 6 4s 4p and are noteworthy for their rela- In 1893, the English physicist Lord Rayleigh noticed 54 0 tive lack of chemical reactivity.