Introduction to Molecular Virology S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Changes to Virus Taxonomy 2004
Arch Virol (2005) 150: 189–198 DOI 10.1007/s00705-004-0429-1 Changes to virus taxonomy 2004 M. A. Mayo (ICTV Secretary) Scottish Crop Research Institute, Invergowrie, Dundee, U.K. Received July 30, 2004; accepted September 25, 2004 Published online November 10, 2004 c Springer-Verlag 2004 This note presents a compilation of recent changes to virus taxonomy decided by voting by the ICTV membership following recommendations from the ICTV Executive Committee. The changes are presented in the Table as decisions promoted by the Subcommittees of the EC and are grouped according to the major hosts of the viruses involved. These new taxa will be presented in more detail in the 8th ICTV Report scheduled to be published near the end of 2004 (Fauquet et al., 2004). Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., and Ball, L.A. (eds) (2004). Virus Taxonomy, VIIIth Report of the ICTV. Elsevier/Academic Press, London, pp. 1258. Recent changes to virus taxonomy Viruses of vertebrates Family Arenaviridae • Designate Cupixi virus as a species in the genus Arenavirus • Designate Bear Canyon virus as a species in the genus Arenavirus • Designate Allpahuayo virus as a species in the genus Arenavirus Family Birnaviridae • Assign Blotched snakehead virus as an unassigned species in family Birnaviridae Family Circoviridae • Create a new genus (Anellovirus) with Torque teno virus as type species Family Coronaviridae • Recognize a new species Severe acute respiratory syndrome coronavirus in the genus Coro- navirus, family Coronaviridae, order Nidovirales -

Identification of Capsid/Coat Related Protein Folds and Their Utility for Virus Classification
ORIGINAL RESEARCH published: 10 March 2017 doi: 10.3389/fmicb.2017.00380 Identification of Capsid/Coat Related Protein Folds and Their Utility for Virus Classification Arshan Nasir 1, 2 and Gustavo Caetano-Anollés 1* 1 Department of Crop Sciences, Evolutionary Bioinformatics Laboratory, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 2 Department of Biosciences, COMSATS Institute of Information Technology, Islamabad, Pakistan The viral supergroup includes the entire collection of known and unknown viruses that roam our planet and infect life forms. The supergroup is remarkably diverse both in its genetics and morphology and has historically remained difficult to study and classify. The accumulation of protein structure data in the past few years now provides an excellent opportunity to re-examine the classification and evolution of viruses. Here we scan completely sequenced viral proteomes from all genome types and identify protein folds involved in the formation of viral capsids and virion architectures. Viruses encoding similar capsid/coat related folds were pooled into lineages, after benchmarking against published literature. Remarkably, the in silico exercise reproduced all previously described members of known structure-based viral lineages, along with several proposals for new Edited by: additions, suggesting it could be a useful supplement to experimental approaches and Ricardo Flores, to aid qualitative assessment of viral diversity in metagenome samples. Polytechnic University of Valencia, Spain Keywords: capsid, virion, protein structure, virus taxonomy, SCOP, fold superfamily Reviewed by: Mario A. Fares, Consejo Superior de Investigaciones INTRODUCTION Científicas(CSIC), Spain Janne J. Ravantti, The last few years have dramatically increased our knowledge about viral systematics and University of Helsinki, Finland evolution. -

Data Mining Cdnas Reveals Three New Single Stranded RNA Viruses in Nasonia (Hymenoptera: Pteromalidae)
Insect Molecular Biology Insect Molecular Biology (2010), 19 (Suppl. 1), 99–107 doi: 10.1111/j.1365-2583.2009.00934.x Data mining cDNAs reveals three new single stranded RNA viruses in Nasonia (Hymenoptera: Pteromalidae) D. C. S. G. Oliveira*, W. B. Hunter†, J. Ng*, Small viruses with a positive-sense single-stranded C. A. Desjardins*, P. M. Dang‡ and J. H. Werren* RNA (ssRNA) genome, and no DNA stage, are known as *Department of Biology, University of Rochester, picornaviruses (infecting vertebrates) or picorna-like Rochester, NY, USA; †United States Department of viruses (infecting non-vertebrates). Recently, the order Agriculture, Agricultural Research Service, US Picornavirales was formally characterized to include most, Horticultural Research Laboratory, Fort Pierce, FL, USA; but not all, ssRNA viruses (Le Gall et al., 2008). Among and ‡United States Department of Agriculture, other typical characteristics – e.g. a small icosahedral Agricultural Research Service, NPRU, Dawson, GA, capsid with a pseudo-T = 3 symmetry and a 7–12 kb USA genome made of one or two RNA segments – the Picor- navirales genome encodes a polyprotein with a replication Abstractimb_934 99..108 module that includes a helicase, a protease, and an RNA- dependent RNA polymerase (RdRp), in this order (see Le We report three novel small RNA viruses uncovered Gall et al., 2008 for details). Pathogenicity of the infections from cDNA libraries from parasitoid wasps in the can vary broadly from devastating epidemics to appar- genus Nasonia. The genome of this kind of virus ently persistent commensal infections. Several human Ј is a positive-sense single-stranded RNA with a 3 diseases, from hepatitis A to the common cold (e.g. -

How Influenza Virus Uses Host Cell Pathways During Uncoating
cells Review How Influenza Virus Uses Host Cell Pathways during Uncoating Etori Aguiar Moreira 1 , Yohei Yamauchi 2 and Patrick Matthias 1,3,* 1 Friedrich Miescher Institute for Biomedical Research, 4058 Basel, Switzerland; [email protected] 2 Faculty of Life Sciences, School of Cellular and Molecular Medicine, University of Bristol, Bristol BS8 1TD, UK; [email protected] 3 Faculty of Sciences, University of Basel, 4031 Basel, Switzerland * Correspondence: [email protected] Abstract: Influenza is a zoonotic respiratory disease of major public health interest due to its pan- demic potential, and a threat to animals and the human population. The influenza A virus genome consists of eight single-stranded RNA segments sequestered within a protein capsid and a lipid bilayer envelope. During host cell entry, cellular cues contribute to viral conformational changes that promote critical events such as fusion with late endosomes, capsid uncoating and viral genome release into the cytosol. In this focused review, we concisely describe the virus infection cycle and highlight the recent findings of host cell pathways and cytosolic proteins that assist influenza uncoating during host cell entry. Keywords: influenza; capsid uncoating; HDAC6; ubiquitin; EPS8; TNPO1; pandemic; M1; virus– host interaction Citation: Moreira, E.A.; Yamauchi, Y.; Matthias, P. How Influenza Virus Uses Host Cell Pathways during 1. Introduction Uncoating. Cells 2021, 10, 1722. Viruses are microscopic parasites that, unable to self-replicate, subvert a host cell https://doi.org/10.3390/ for their replication and propagation. Despite their apparent simplicity, they can cause cells10071722 severe diseases and even pose pandemic threats [1–3]. -

Deciphering the Virome of Culex Vishnui Subgroup Mosquitoes, the Major Vectors of Japanese Encephalitis, in Japan
viruses Article Deciphering the Virome of Culex vishnui Subgroup Mosquitoes, the Major Vectors of Japanese Encephalitis, in Japan Astri Nur Faizah 1,2 , Daisuke Kobayashi 2,3, Haruhiko Isawa 2,*, Michael Amoa-Bosompem 2,4, Katsunori Murota 2,5, Yukiko Higa 2, Kyoko Futami 6, Satoshi Shimada 7, Kyeong Soon Kim 8, Kentaro Itokawa 9, Mamoru Watanabe 2, Yoshio Tsuda 2, Noboru Minakawa 6, Kozue Miura 1, Kazuhiro Hirayama 1,* and Kyoko Sawabe 2 1 Laboratory of Veterinary Public Health, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan; [email protected] (A.N.F.); [email protected] (K.M.) 2 Department of Medical Entomology, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo 162-8640, Japan; [email protected] (D.K.); [email protected] (M.A.-B.); k.murota@affrc.go.jp (K.M.); [email protected] (Y.H.); [email protected] (M.W.); [email protected] (Y.T.); [email protected] (K.S.) 3 Department of Research Promotion, Japan Agency for Medical Research and Development, 20F Yomiuri Shimbun Bldg. 1-7-1 Otemachi, Chiyoda-ku, Tokyo 100-0004, Japan 4 Department of Environmental Parasitology, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8510, Japan 5 Kyushu Research Station, National Institute of Animal Health, NARO, 2702 Chuzan, Kagoshima 891-0105, Japan 6 Department of Vector Ecology and Environment, Institute of Tropical Medicine, Nagasaki University, 1-12-4 Sakamoto, Nagasaki 852-8523, Japan; [email protected] -

Tically Expands Our Understanding on Virosphere in Temperate Forest Ecosystems
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 21 June 2021 doi:10.20944/preprints202106.0526.v1 Review Towards the forest virome: next-generation-sequencing dras- tically expands our understanding on virosphere in temperate forest ecosystems Artemis Rumbou 1,*, Eeva J. Vainio 2 and Carmen Büttner 1 1 Faculty of Life Sciences, Albrecht Daniel Thaer-Institute of Agricultural and Horticultural Sciences, Humboldt-Universität zu Berlin, Ber- lin, Germany; [email protected], [email protected] 2 Natural Resources Institute Finland, Latokartanonkaari 9, 00790, Helsinki, Finland; [email protected] * Correspondence: [email protected] Abstract: Forest health is dependent on the variability of microorganisms interacting with the host tree/holobiont. Symbiotic mi- crobiota and pathogens engage in a permanent interplay, which influences the host. Thanks to the development of NGS technol- ogies, a vast amount of genetic information on the virosphere of temperate forests has been gained the last seven years. To estimate the qualitative/quantitative impact of NGS in forest virology, we have summarized viruses affecting major tree/shrub species and their fungal associates, including fungal plant pathogens, mutualists and saprotrophs. The contribution of NGS methods is ex- tremely significant for forest virology. Reviewed data about viral presence in holobionts, allowed us to address the role of the virome in the holobionts. Genetic variation is a crucial aspect in hologenome, significantly reinforced by horizontal gene transfer among all interacting actors. Through virus-virus interplays synergistic or antagonistic relations may evolve, which may drasti- cally affect the health of the holobiont. Novel insights of these interplays may allow practical applications for forest plant protec- tion based on endophytes and mycovirus biocontrol agents. -

Caracterización Genómica Y Biológica De Un Nuevo Cheravirus En Babaco (Vasconcellea X Heilbornii
1 “Caracterización genómica y biológica de un nuevo Cheravirus en babaco (Vasconcellea x heilbornii. var. pentagona)” Salazar Maldonado, Liseth Carolina Departamento de Ciencias de la Vida y de la Agricultura Carrera de Ingeniería en Biotecnología Trabajo de titulación, previo a la obtención del título de Ingeniera en Biotecnología Flores Flor, Francisco Javier PhD. 9 de octubre del 2020 2 Resultado del análisis de Urkund 3 4 Departamento de Ciencias de la Vida y de la Agricultura Carrera de Ingeniería en Biotecnología Certificación 5 Departamento de Ciencias de la Vida y de la Agricultura Carrera de Ingeniería en Biotecnología Responsabilidad de autoría 6 Departamento de Ciencias de la Vida y de la Agricultura Carrera de Ingeniería en Biotecnología Autorización de publicación 7 Dedicatoria Dedico este trabajo a mi madre Silvia Maldonado, a mis abuelitos y a mi tío Fabián, quienes procuraron que no me falte nada durante el transcurso de la carrera universitaria para que así todo mi esfuerzo y concentración se centre en mis estudios. 8 Agradecimiento Agradezco a mi familia por todo el apoyo y palabras de aliento en los momentos difíciles, este logro también es de ustedes. A todos mis familiares de Yaguachi que me acogieron durante mi estancia en la costa y me hicieron sentir como en casa. A la Licenciada Carmen Plaza por recibirme en su hogar durante los meses que estuve en Guayaquil. Agradezco a mi tutor el Dr. Francisco Flores por darme todas las facilidades para culminar este proyecto y por guiarme en el transcurso de su realización. Al Dr. Diego Quito por haberme dado la apertura para la realización del proyecto y por tomarse el tiempo de compartir su conocimiento y ayudarme a despejar todas mis dudas. -

Taxonomy of Picornaviridae: Current Situation and Future Proposals
EUROPIC 2008 TaxonomyTaxonomy ofof PicornaviridaePicornaviridae:: C1 CurrentCurrent SituationSituation andand FutureFuture ProposalsProposals Nick J. Knowles1, Tapani Hovi2, Timo Hyypiä3, Andrew M.Q. King4, A. Michael Lindberg5, Philip D. Minor6, Mark A. Pallansch7, Ann C. Palmenberg8, Tim Skern9, Glyn Stanway10, Teruo Yamashita11 and Roland Zell12 (1) Institute for Animal Health, Pirbright, UK; (2) Enterovirus Laboratory, KTL, Helsinki, Finland; (3) Dept. of Virology, Univ. Turku, Finland; 4) ‘Sunfield’, Dawney Hill, Pirbright, Woking, Surrey, UK; (5) School of Pure and Applied Natural Sciences, University of Kalmar, Sweden; (6) NIBSC, South Mimms, UK; (7) CDC, Atlanta, USA; (8) Institute for Molecular Virology, Wisconsin, USA; (9) Max F. Perutz Laboratories, Medical Univ. Vienna, Austria; (10) Dept. of Biological Sci., Univ. Essex, UK; (11) Aichi Prefectural Institute of Public Health, Aichi, Japan; (12) Institut fuer Virologie und Antivirale Therapie, Jena, Germany. RECENT ICTV‐APPROVED CHANGES (ICTV LEVEL 05) “Sapelovirus” Teschovirus Porcine teschovirus "Avian sapelovirus" Four taxonomic proposals have recently been approved by the ICTV: i) to combine the "Porcine sapelovirus" "Human rhinovirus C" "Simian sapelovirus" Cardiovirus Enterovirus and Rhinovirus genera, keeping the existing name Enterovirus; ii) to combine Encephalomyocarditis virus the species Poliovirus and Human enterovirus C, retaining the latter name; iii) to assign Human rhinovirus A Theilovirus Human enterovirus C as the type species of the enterovirus genus; iv) -

Complete Nucleotide Sequence, Molecular Analysis and Genome Structure of Bacteriophage A118 of Listeria Monocytogenes : Implications for Phage Evolution
Molecular Microbiology (2000) 35(2), 324±340 Complete nucleotide sequence, molecular analysis and genome structure of bacteriophage A118 of Listeria monocytogenes : implications for phage evolution Martin J. Loessner,1* Ross B. Inman,2 Peter Lauer3 local, but sometimes extensive, similarities to a num- and Richard Calendar3 ber of phages spanning a broader phylogenetic range 1Institut fuÈr Mikrobiologie, FML Weihenstephan, of various low GC host bacteria, which implies rela- Technische UniversitaÈtMuÈnchen, Weihenstephaner tively recent exchange of genes or genetic modules. Berg 3, 85350 Freising, Germany. We have also identi®ed the A118 attachment site 2Institute for Molecular Virology, University of attP and the corresponding attB in Listeria monocyto- Wisconsin at Madison, 1525 Linden Drive, Madison, genes, and show that site-speci®c integration of the Wisconsin 53706, USA. A118 prophage by the A118 integrase occurs into a 3Department of Molecular and Cell Biology, host gene homologous to comK of Bacillus subtilis, University of California at Berkeley, 401 Barker Hall, an autoregulatory gene specifying the major compe- Berkeley, California 94720-3202, USA. tence transcription factor. Summary Introduction A118 is a temperate phage isolated from Listeria Listeria monocytogenes is a non-spore-forming, opportu- monocytogenes. In this study, we report the entire nistic Gram-positive pathogen, responsible for severe nucleotide sequence and structural analysis of its infections in both animals and humans, which is almost 40 834 bp DNA. Electron microscopic and enzymatic exclusively transmitted via contaminated food. Recurrent analyses revealed that the A118 genome is a linear, outbreaks of Listeriosis (CDC, 1998; Slutsker and Schuchat, circularly permuted, terminally redundant collection 1999) have emphasized the need for a better understand- of double-stranded DNA molecules. -

Discrimination and Trancriptional Response
DISCRIMINATION AND TRANCRIPTIONAL RESPONSE ANALYSIS OF HEMIPTERAN PHYTOPATHOGEN VECTORS By SHARON ANDREASON Bachelor of Science in Biology University of Texas at Tyler Tyler, Texas 2009 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY July, 2016 DISCRIMINATION AND TRANSCRIPTIONAL RESPONSE ANALYSIS OF HEMIPTERAN PHYTOPATHOGEN VECTORS Dissertation Approved: Astri Wayadande, Ph.D. Dissertation Adviser Jacqueline Fletcher, Ph.D. Francisco Ochoa-Corona, Ph.D. Ulrich Melcher, Ph.D. ii ACKNOWLEDGEMENTS I would first like to extend my deepest thanks to my advisor, Dr. Astri Wayadande, for all of her years of support. Always full of positivity, encouragement, and advice, her enthusiasm for research and effort devoted to others are attributes that I aspire to cultivate in my future career. I am forever grateful for her guidance throughout earning my degree. I would also like to thank my committee members, Drs. Jacqueline Fletcher, Francisco Ochoa-Corona, and Ulrich Melcher, each of whom have not only offered me thoughtful advice through my research, but have also educated and inspired me in unique and invaluable ways. I am deeply appreciative of their guidance over the years. I want to thank Dr. Judith Brown, without whom I would not have had the opportunity to work on such an important vector system. I am sincerely grateful to Dr. Mohammad Arif, who provided exceptional expertise and advice through my whitefly projects. I would also like to extend my thanks to Dr. Trenna Blagden for all her help and guidance on my projects, particularly during my work with EDNA, as well as for the professional advice she has provided. -

Chirico Et Al. Supplementary Methods and Results
!"#$#%&'()'*+,'-.//+(0(1)*$2'3()"&45'*14'6(5.+)5' ! 7*8+('-9,'"#$%!&$'()*!+,#-)$.!.-+.+-/(+%0!$%1!2$.0(1!1#02-(./(+%' ! Taxon Total Validated Species Genome length Overlap Capsid type Capsid Species Species with (ln) proportion flexible? overlap (ln) DNA viruses Acanthamoeba-polyphaga-mimivirus 1 0 Adenoviridae 44 12 12 10.47 -3.71 icosahedral no Anellovirus 5 1 1 8.26 -1.78 icosahedral no Ascoviridae 3 0 Asfarviridae 1 0 Bacillus-phage-GIL-sixteen-c 1 1 1 9.61 -3.05 no description ? Bacillus-virus-one 1 0 Baculoviridae 43 1 1 11.78 -4.79 rod shaped yesa Bicaudaviridae 2 0 Circoviridae 16 3 3 7.65 -1.78 icosahedral no Clostridium-phage-phiC-two 1 0 Corticovirus 1 1 1 9.22 -4.76 icosahedral no Fuselloviridae 5 3 3 9.69 -3.22 lemon-shaped yesb Geminiviridae 199 82 80 8.23 -1.54 icosahedral no Geobacillus-phage-GBSVone 1 1 1 10.45 -4.69 no description ? Globuloviridae 2 0 Gryllus-bimaculatus-nudivirus 1 0 Heliothis-zea-virus-one 1 0 Herpesviridae 47 26 26 11.97 -4.44 icosahedral no His-one-virus 1 0 His-two-virus 1 0 Inoviridae 25 18 17 8.88 -4.64 filamentous yes Iridoviridae 8 1 1 11.54 -5.31 icosahedral no Lipothrixviridae 8 2 2 10.62 -4.34 rod shaped yes Microviridae 55 13 12 8.56 -2.23 icosahedral no Myoviridae 71 35 35 11.37 -4.89 icosahedral no Nanoviridae 6 1 0 Nimaviridae 1 0 Papillomaviridae 66 13 13 8.97 -3.11 icosahedral no Parvoviridae 44 8 6 8.56 -2.14 icosahedral no Phycodnaviridae 8 1 1 12.72 -5.95 icosahedral no Plasmaviridae 1 1 1 9.39 -8.00 quasi-spherical yes Podoviridae 62 32 32 10.59 -3.58 icosahedral no Polydnaviridae -
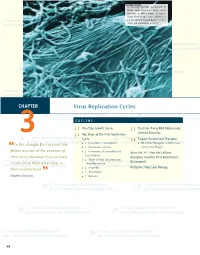
Virus Replication Cycles
© Jones and Bartlett Publishers. NOT FOR SALE OR DISTRIBUTION A scanning electron micrograph of Ebola virus particles. Ebola virus contains an RNA genome. It causes Ebola hemorrhagic fever, which is a severe and often fatal disease in hu- mans and nonhuman primates. CHAPTER Virus Replication Cycles OUTLINE 3.1 One-Step Growth Curves 3.3 The Error-Prone RNA Polymerases: 3 3.2 Key Steps of the Viral Replication Genetic Diversity Cycle 3.4 Targets for Antiviral Therapies In the struggle for survival, the ■ 1. Attachment (Adsorption) ■ RNA Virus Mutagens: A New Class “ ■ 2. Penetration (Entry) of Antiviral Drugs? fi ttest win out at the expense of ■ 3. Uncoating (Disassembly and Virus File 3-1: How Are Cellular Localization) their rivals because they succeed Receptors Used for Viral Attachment ■ 4. Types of Viral Genomes and Discovered? in adapting themselves best to Their Replication their environment. ■ 5. Assembly Refresher: Molecular Biology ” ■ 6. Maturation Charles Darwin ■ 7. Release 46 229329_CH03_046_069.indd9329_CH03_046_069.indd 4466 11/18/08/18/08 33:19:08:19:08 PPMM © Jones and Bartlett Publishers. NOT FOR SALE OR DISTRIBUTION CASE STUDY The campus day care was recently closed during the peak of the winter fl u season because many of the young children were sick with a lower respiratory tract infection. An email an- nouncement was sent to all students, faculty, and staff at the college that stated the closure was due to a metapneumovirus outbreak. The announcement briefed the campus com- munity with information about human metapneumonoviruses (hMPVs). The announcement stated that hMPV was a newly identifi ed respiratory tract pathogen discovered in the Netherlands in 2001.