The Raphe-Hippocampal Tract and Its Age Differences: Diffusion Tensor Imaging and Probabilistic Tractography Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neural Mechanisms of Oxytocin and Serotonin Interaction in Non-Human Primates and Patients with Autism Arthur Lefevre
Neural mechanisms of oxytocin and serotonin interaction in non-human primates and patients with autism Arthur Lefevre To cite this version: Arthur Lefevre. Neural mechanisms of oxytocin and serotonin interaction in non-human primates and patients with autism. Neuroscience. Université de Lyon, 2016. English. NNT : 2016LYSE1323. tel-01508642 HAL Id: tel-01508642 https://tel.archives-ouvertes.fr/tel-01508642 Submitted on 14 Apr 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N°d’ordre NNT : xxx THESE de DOCTORAT DE L’UNIVERSITE DE LYON opérée au sein de l’Université Claude Bernard Lyon 1 Ecole Doctorale 476 (Neuroscience et Cognition) Spécialité de doctorat : Discipline : Neurosciences Soutenue publiquement le 13 Décembre 2016, par : Arthur LEFEVRE __________________________________________ Neural mechanisms of oxytocin and serotonin interaction in non- human primates and patients with autism __________________________________________ Thèse dirigée par Angela Sirigu (DRCE, CNRS) Devant le jury composé de : GERVAIS Rémi, Président, (Professeur à l’Université Lyon 1 Claude Bernard) CHINI BICE, Rapporteur, (Senior researcher at the Institute of Neuroscience, Milan) CHAKRABARTI Bhismadev, Rapporteur, (Associate Professor at university of Reading and Senior researcher at Cambridge university) KRAUS Christoph, Examinateur, (Senior researcher at the Medical university of Vienna) SIRIGU Angela, Directrice de thèse, (DRCE, Université de Lyon, CNRS) 1 UNIVERSITE CLAUDE BERNARD - LYON 1 Président de l’Université M. -

Dopamine and Serotonin Metabolism in Parkinsonian Models
Dopamine and Serotonin Metabolism in Parkinsonian Models Carmen de la Fuente Barrigon UCL Great Ormond Street Institute of Child Health Thesis submitted for the degree of Doctor of Philosophy (PhD) awarded by University College London (UCL). Funded by Marie Skłodowska-Curie Actions of the European Union’s Seventh Framework Programme (FP7). APRIL 2018 1 I, Carmen de la Fuente Barrigon confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Signed ………………………………………….. Date ……………………………………………… 3 Dedico esta tesis a los pilares de mi vida. A mis padres, Tomás y Marisa. A mi hermana, Paloma. A mi amor, Francesco. Por vuestros sacrificios, paciencia, amor y apoyo incondicionales que me hacen ser quien soy hoy. 5 Abstract Parkinson’s disease (PD) is a neurodegenerative disorder caused by loss of dopaminergic neurons in the substantia nigra. Different pathogenic mechanisms have been implicated, including loss of mitochondrial complex I function and dysfunction of lysosomal glucocerebrosidase (GBA1) (Neumann et al., 2009; Schapira et al., 1990). Also, it has been hypothesised that serotonin metabolism could be affected in these patients due to the number of enzymes shared by both pathways (Albizu et al., 2011). This thesis considers the potential involvement of complex I and GBA1 in PD using HPLC analysis of changes in the extracellular levels of the metabolites of dopamine and serotonin, and the expression and activity of the enzymes of the dopamine pathway. Using SH-SY5Y cells, complex I deficiency was modelled using rotenone, and GBA1 deficiency was modelled using conduritol B epoxide (CBE). -

Activation of Raphe Nuclei Triggers Rapid and Distinct Effects on Parallel Olfactory Bulb Output Channels
Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kapoor, Vikrant, Allison Provost, Prateek Agarwal, and Venkatesh N. Murthy. 2015. “Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels.” Nature neuroscience 19 (2): 271-282. doi:10.1038/nn.4219. http:// dx.doi.org/10.1038/nn.4219. Published Version doi:10.1038/nn.4219 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:29002684 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Nat Neurosci Manuscript Author . Author manuscript; Manuscript Author available in PMC 2016 August 01. Published in final edited form as: Nat Neurosci. 2016 February ; 19(2): 271–282. doi:10.1038/nn.4219. Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels Vikrant Kapoor1,2,†, Allison Provost1,2, Prateek Agarwal1,2, and Venkatesh N. Murthy1,2,† 1Center for Brain Science, Harvard University, Cambridge, MA 02138 2Department of Molecular & Cellular Biology, Harvard University, Cambridge, MA 02138 Abstract The serotonergic raphe nuclei are involved in regulating brain states over time-scales of minutes and hours. We examined more rapid effects of serotonergic activation on two classes of principal neurons in the mouse olfactory bulb, mitral and tufted cells, which send olfactory information to distinct targets. -

Scaling Our World View: How Monoamines Can Put Context Into Brain Circuitry
HYPOTHESIS AND THEORY published: 20 December 2018 doi: 10.3389/fncel.2018.00506 Scaling Our World View: How Monoamines Can Put Context Into Brain Circuitry Philipp Stratmann 1,2*, Alin Albu-Schäffer 1,2 and Henrik Jörntell 3 1 Sensor Based Robotic Systems and Intelligent Assistance Systems, Department of Informatics, Technical University of Munich, Garching, Germany, 2 German Aerospace Center (DLR), Institute of Robotics and Mechatronics, Weßling, Germany, 3 Neural Basis of Sensorimotor Control, Department of Experimental Medical Science, Lund University, Lund, Sweden Monoamines are presumed to be diffuse metabotropic neuromodulators of the topographically and temporally precise ionotropic circuitry which dominates CNS functions. Their malfunction is strongly implicated in motor and cognitive disorders, but their function in behavioral and cognitive processing is scarcely understood. In this paper, the principles of such a monoaminergic function are conceptualized for locomotor control. We find that the serotonergic system in the ventral spinal cord scales ionotropic signals and shows topographic order that agrees with differential gain modulation of ionotropic subcircuits. Whereas the subcircuits can collectively signal predictive models of the world based on life-long learning, their differential scaling continuously adjusts these models to changing mechanical contexts based on sensory input on a fast time scale of a few 100 ms. The control theory of biomimetic robots demonstrates that this precision scaling is an effective and resource-efficient -

Context-Dependent Modulation of Auditory Processing by Serotonin
Hearing Research 279 (2011) 74e84 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Context-dependent modulation of auditory processing by serotonin L.M. Hurley a,*, I.C. Hall b a Indiana University, Jordan Hall/Biology, 1001 E. Third St, Bloomington, IN 47405, USA b Columbia University, 901 Fairchild Center, M.C. 2430, New York, NY 10027, USA article info abstract Article history: Context-dependent plasticity in auditory processing is achieved in part by physiological mechanisms that Received 3 October 2010 link behavioral state to neural responses to sound. The neuromodulator serotonin has many character- Received in revised form istics suitable for such a role. Serotonergic neurons are extrinsic to the auditory system but send 13 December 2010 projections to most auditory regions. These projections release serotonin during particular behavioral Accepted 20 December 2010 contexts. Heightened levels of behavioral arousal and specific extrinsic events, including stressful or Available online 25 December 2010 social events, increase serotonin availability in the auditory system. Although the release of serotonin is likely to be relatively diffuse, highly specific effects of serotonin on auditory neural circuitry are achieved through the localization of serotonergic projections, and through a large array of receptor types that are expressed by specific subsets of auditory neurons. Through this array, serotonin enacts plasticity in auditory processing in multiple ways. Serotonin changes the responses of auditory neurons to input through the alteration of intrinsic and synaptic properties, and alters both short- and long-term forms of plasticity. The infrastructure of the serotonergic system itself is also plastic, responding to age and cochlear trauma. -

Overview of the Reticular Formation (RF)
TeachSheet Reticular Formation & Diffuse modulatory system Overview of the Reticular Formation (RF) The term reticular formation refers to the neuronal network within the brainstem, although it continues rostrally into the thalamus and hypothalamus and caudally into the propriospinal network of the spinal cord. A “coordinating system” (like the Limbic system) with “connections” to sensory, somatic motor and visceral motor systems Organization can be subdivided into two neuronal cell “columns” (medial to lateral) as well as on the basis of their neurotransmitter release Neuronal columns (many nuclei and names, but these are some of the major ones): o “Medial tegmental field” (large-celled nuclei) . Origin of the reticulospinal pathway . Role in coordinating posture, eye and head movements. o “Lateral tegmental field” (smaller and fewer cells, shorter local projections) . Extends from the medulla to the pons . Coordinate autonomic and limbic functions (e.g. micturition, swallowing, mastication and vocalization) The functions of the reticular formation include their ability to coordinate motor and sensory brainstem nuclei: o Pattern generator . Eye movements; horizontal (PPRF) and vertical (riMLF) . Rhythmical chewing movements (pons) . Posture and locomotion (midbrain and pons) . Swallowing, vomiting, coughing and sneezing (medulla) . Micturition (pons) o Respiratory control (medulla); expiratory, inspiratory, apneustic and pneumotaxic o Cardiovascular control (medulla); vasomotor pressor/depressor, cardioacceleratory and inhibitory. Afferents arise from baroreceptors (carotid sinus and aortic arch), chemoreceptors (carotid sinus, lateral reticular formation chemosensitive area in the medulla) and stretch receptors (lung and respiratory muscles) . Efferents arise from reticular formation neurons within the pons and medulla o Sensory modulation or “gate” control . The term “gating” refers to “modulation” of synaptic transmission from one set of neurons to the next. -

Sclocco Brainstim2019.Pdf
Brain Stimulation xxx (xxxx) xxx Contents lists available at ScienceDirect Brain Stimulation journal homepage: http://www.journals.elsevier.com/brain-stimulation The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: A multimodal ultrahigh-field (7T) fMRI study * Roberta Sclocco a, b, , Ronald G. Garcia a, c, Norman W. Kettner b, Kylie Isenburg a, Harrison P. Fisher a, Catherine S. Hubbard a, Ilknur Ay a, Jonathan R. Polimeni a, Jill Goldstein a, c, d, Nikos Makris a, c, Nicola Toschi a, e, Riccardo Barbieri f, g, Vitaly Napadow a, b a Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, USA b Department of Radiology, Logan University, Chesterfield, MO, USA c Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA d Department of Obstetrics and Gynecology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA e Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy f Department of Electronics, Information and Bioengineering, Politecnico di Milano, Italy g Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA article info abstract Article history: Background: Brainstem-focused mechanisms supporting transcutaneous auricular VNS (taVNS) effects Received 12 September 2018 are not well understood, particularly in humans. We employed ultrahigh field (7T) fMRI and evaluated Received in revised form the influence of respiratory phase for optimal targeting, applying our respiratory-gated auricular vagal 2 January 2019 afferent nerve stimulation (RAVANS) technique. Accepted 6 February 2019 Hypothesis: We proposed that targeting of nucleus tractus solitarii (NTS) and cardiovagal modulation in Available online xxx response to taVNS stimuli would be enhanced when stimulation is delivered during a more receptive state, i.e. -

Brain Structure and Function Related to Headache
Review Cephalalgia 0(0) 1–26 ! International Headache Society 2018 Brain structure and function related Reprints and permissions: sagepub.co.uk/journalsPermissions.nav to headache: Brainstem structure and DOI: 10.1177/0333102418784698 function in headache journals.sagepub.com/home/cep Marta Vila-Pueyo1 , Jan Hoffmann2 , Marcela Romero-Reyes3 and Simon Akerman3 Abstract Objective: To review and discuss the literature relevant to the role of brainstem structure and function in headache. Background: Primary headache disorders, such as migraine and cluster headache, are considered disorders of the brain. As well as head-related pain, these headache disorders are also associated with other neurological symptoms, such as those related to sensory, homeostatic, autonomic, cognitive and affective processing that can all occur before, during or even after headache has ceased. Many imaging studies demonstrate activation in brainstem areas that appear specifically associated with headache disorders, especially migraine, which may be related to the mechanisms of many of these symptoms. This is further supported by preclinical studies, which demonstrate that modulation of specific brainstem nuclei alters sensory processing relevant to these symptoms, including headache, cranial autonomic responses and homeostatic mechanisms. Review focus: This review will specifically focus on the role of brainstem structures relevant to primary headaches, including medullary, pontine, and midbrain, and describe their functional role and how they relate to mechanisms -

Neurophysiological and Neurohumoral Changes by High Frequency Stimulation (HFS) of Nucleus Accumbens (Nac)
From the Department of Neurosurgery of the University of Luebeck Director: Prof. Dr. med. Volker Tronnier Neurophysiological and neurohumoral changes by High Frequency Stimulation (HFS) of Nucleus Accumbens (NAc) Dissertation for Fulfillment of Requirements for the Doctoral Degree of the University of Luebeck from the Department of Natural Sciences Submitted by Ramya Varatharajan Thirugnanasambantham Born in Veeramanipatti-TN, India Luebeck, 2015 I Ramya Varatharajan Thirugnanasambantham Department of Neurosurgery University of Luebeck Ratzeburger allee 160 23562, Luebeck Germany First referee: Prof. Dr. Volker Tronnier Second referee: Prof. Dr. Thomas Martinetz Date of oral examination: 23.09.2015 Approved for printing, Luebeck: 30.09.2015 II Acknowledgements The hard work of so many people is involved in successful completion of this thesis, that a genuine list is virtually impossible. I convey my thanks and gratitude to Prof. Dr. med. Volker Tronnier, Department of Neurosurgery, for providing this opportunity to work under his guidance with this interesting topic. I express my deepest gratitude to Prof. Dr. med. Andreas Moser and Prof. Dr. rer. nat. Ulrich G. Hofmann for providing Laboratory Infrastructure, financial and moral support. Beyond scientific supervision, I was lucky to have the people from liquorlab, guiding me through all matters during my stay in Luebeck. I am thankful to Katharina Schnackenberg whose excellent experience in the laboratory and generous practical help was essential for the successful work at the lab. Thanks to Karin Wiegers, Marie Luis Reher and Gisa Brunk for being like friends and taking good care of me. I am very much thankful to Krishnakumar, Kevin Joseph, Sonya Neto, Mehrnaz Hazrati, Yijing Xie and Susanne Loeffler for their support, encouragement and timely suggestions. -

Imaging Elevated Brain Arachidonic Acid Signaling in Unanesthetized Serotonin Transporter (5-HTT)-Deficient Mice
Neuropsychopharmacology (2009) 34, 1695–1709 & 2009 Nature Publishing Group All rights reserved 0893-133X/09 $32.00 www.neuropsychopharmacology.org Imaging Elevated Brain Arachidonic Acid Signaling in Unanesthetized Serotonin Transporter (5-HTT)-Deficient Mice Mireille Basselin*,1, Meredith A Fox2, Lisa Chang1, Jane M Bell1, Dede Greenstein3, Mei Chen1, 2 1 Dennis L Murphy and Stanley I Rapoport 1Brain Physiology and Metabolism Section, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA; 2Laboratory of Clinical Science, National Institutes of Health, Bethesda, MD, USA; 3Child Psychiatry Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA Certain polymorphisms reduce serotonin (5-HT) reuptake transporter (5-HTT) function and increase susceptibility to psychiatric +/À disorders. Heterozygous (5-HTT )-deficient mice, models for humans with these polymorphisms, have elevated brain 5-HT concentrations and behavioral abnormalities. As postsynaptic 5-HT2A/2C receptors are coupled to cytosolic phospholipase A2 (cPLA2), which releases arachidonic acid (AA) from membrane phospholipid, 5-HTT-deficient mice may have altered brain AA signaling and metabolism. To test this hypothesis, signaling was imaged as an AA incorporation coefficient k* in unanesthetized homozygous knockout À/À +/À +/+ (5-HTT ), 5-HTT and wild-type (5-HTT ), mice following saline (baseline) or 1.5 mg/kg s.c. DOI, a partial 5-HT2A/2C receptor agonist. Enzyme activities, metabolite concentrations, and head-twitch responses to DOI were also measured. Baseline k* was widely +/À À/À +/+ +/+ elevated by 20–70% in brains of 5-HTT and 5-HTT compared to 5-HTT mice. DOI increased k* in 5-HTT mice, but decreased k* in 5-HTT-deficient mice. -

Analysis of Pacemaker Activity in a Two-Component Model of Some
Analysis of pacemaker activity in a two-component model of some brainstem neurons Henry C. Tuckwell1,2∗, Ying Zhou3,, Nicholas J. Penington 4,5 1 School of Electrical and Electronic Engineering, University of Adelaide, Adelaide, South Australia 5005, Australia 2 School of Mathematical Sciences, Monash University, Clayton, Victoria 3800, Australia 3 Department of Mathematics, Lafayette College, 1 Pardee Drive, Easton, PA 18042, USA 4 Department of Physiology and Pharmacology, 5 Program in Neural and Behavioral Science and Robert F. Furchgott Center for Neural and Behavioral Science State University of New York, Downstate Medical Center, Box 29, 450 Clarkson Avenue, Brooklyn, NY 11203-2098, USA ∗ Corresponding author: Henry C. Tuckwell, School of Electrical and Electronic Engineering, University of Adelaide, North Terrace, Adelaide, SA 5005, Australia; Tel: 61-481192816; Fax: 61-8-83134360: email: [email protected] September 11, 2018 arXiv:1704.03941v2 [q-bio.NC] 15 Apr 2017 1 Abstract Serotonergic, noradrenergic and dopaminergic brainstem (including midbrain) neu- rons, often exhibit spontaneous and fairly regular spiking with frequencies of order a few Hz, though dopaminergic and noradrenergic neurons only exhibit such pacemaker- type activity in vitro or in vivo under special conditions. A large number of ion channel types contribute to such spiking so that detailed modeling of spike generation leads to the requirement of solving very large systems of differential equations. It is use- ful to have simplified mathematical models of spiking in such neurons so that, for example, features of inputs and output spike trains can be incorporated including stochastic effects for possible use in network models. -
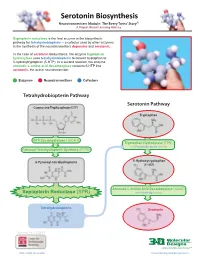
Serotonin Biosynthesis Neurotransmitters Module: the Beery Twins’ Story© a Project-Based Learning Activity
Serotonin Biosynthesis Neurotransmitters Module: The Beery Twins’ Story© A Project-Based Learning Activity Sepiapterin reductase is the final enzyme in the biosynthetic pathway for tetrahydrobiopterin – a cofactor used by other enzymes in the synthesis of the neurotransmitters dopamine and serotonin. In the case of serotonin biosynthesis, the enzyme tryptophan hydroxylase uses tetrahydrobiopterin to convert tryptophan to 5-hydroxytryptophan (5-HTP). In a second reaction, the enzyme aromatic L-amino acid decarboxylase converts 5-HTP into serotonin, the active neurotransmitter. Enzymes Neurotransmitters Cofactors Tetrahydrobiopterin Pathway Serotonin Pathway Guanosine Triphosphate (GTP) Tryptophan GTP Cyclohydrolase I (GCH1) Tryptophan Hydroxylase (TPH) with Tetrahydrobiopterin Cofactor Pyruvoyl-Tetrahydropterin Synthase (PTPS) 6-Pyruvoyl-tetrahydropterin 5-Hydroxytryptophan (5-HTP) Aromatic L-Amino Acid Decarboxylase (AAAD) Sepiapterin Reductase (SPR) with Vitamin B6 Cofactor Tetrahydrobiopterin Serotonin Version 1.4 -12/2015 ...where molecules become real TM http://cbm.msoe.edu www.3dmoleculardesigns.com Serotonin Biosynthesis Model Guide Neurotransmitters Module: The Beery Twins’ Story© A Project-Based Learning Activity TPH AAAD Tryptophan 5-Hydroxytryptophan Serotonin Tryptophan (Trp or W) is one of the 20 standard 5-Hydroxytryptophan, an intermediate The nal step in the serotonin biosynthesis amino acids and is an essential amino acid that molecule in the serotonin biosynthesis pathway requires the removal of the cannot be synthesized by the human body. pathway, is formed by the addition of a carboxylic acid group (COOH) from the Tryptophan is composed of the standard amino hydroxyl (OH) group to the fth carbon of the backbone of 5-hydroxytryptophan to form acid backbone with an indole ring side chain. indole ring of tryptophan.