The Future of Chlorine Disinfectant Choice in Rural Areas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Baseline St1udy Training in Sea Safety Development
BASELINE ST1UDY FOR TRAINING IN SEA SAFETY DEVELOPMENT PROGRAMME IN EAST GODAVARI DISTRICT, ANDHRA. PRADESH NINA FOR FOOD AND AGRICULTURE ORGANISATION OF TFIE UNITED NATIONS AND DEPARTMENT OF FISHERIES, GOVTOF ANDHRAPRADESH BY ACTION FOR FOOD PRODUCTION (AFPRO) FIELD UNIT VI, HYDERABAD 1998 TRAINING IN SEA SAFETY DEVELOPIVIENT PROGRAMME IN EAST GODA VARI DISTRICT, ANDHRA PRADESH INDIA TCP/IND/6712 BASELINE STUDY November, 1997January, 1998 BY ACTION FOR FOOD PRODUCTION (AFPRO) FIELD UNIT VI, HYDERABAD 12-13-483/39, Street No.1, Tarnaka Secunderabad - 500 017 DEPARTMENT OF FISHERIES, GOVT.OF ANDHRAPRADESH FOOD AND AGRICULTURE ORGANISATION OF THE UNITED NATIONS The designations employed and the presentations of the material in this document do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. ACKNOWLEDGMENTS Action for Food Production (AFPRO) Field Unit VI is grateful to the Food and Agriculture Organisation of the United Nations (FAO) and the Department of Fisheries Andhra Pradesh for giving the opportunityfor conducting the Baseline Study - Training in Sea Safety Development Programme in East Godavari District, Andhra Pradesh. Action for Food Production (AFPRO) Field Unit VI wish to thank the following for all the assistance and cooperation extended during the study. The Fisherfolk and Sarpanches of Balusitippa, Bhairavapalem and other villages (Mansanitippa, Komaragiri, Joggampetta, Gadimoga, Peddavalsula). Mr.O. Bhavani Shankar,Additional Director and Conunisioner ofFisheries in Charge, Hyderabad. Mr.Ch.Krishna Murthy, Joint Director of Fisheries, Hyderabad. -

Missing Person - Period Wise Report (CIS) 26/02/2019 Page 1 of 50
Missing Person - Period Wise Report (CIS) 26/02/2019 Page 1 of 50 Crime No., U/S, PS, Name District 31/2019 for U/S Boy,Girl,Woman-Missing Person of the case of Tadikalapudi PS, West Godavari Dst, Andhra Name Polimetla Manohar Father Name Gopala Krishna Gender Male Age 3 Age Missing Date 26-01-2019 Missing from Location Contact Phone 0 Jogadigudem H/o Kamavarapukota,Jogadigudem H/o Kamavarapukota,Kamavarapukota, West Godavari, Contact Address Andhra Pradesh Languages Known Approx. Height 2.5 Hair Back Combed Complexion Brownish Dark Built Normal ID Marks - Articles Found Mental Condition Date of FIR 26/01/2019 PS Phone - Brief Facts of the Case Occurred on 11-01-2019 at 3-30 PM, at, Ch.Pothepalli (V), Dwaraka Tirumala (M) and same was reported on 26-01-2019 at 5-30 PM., wherein the complainant Metabalimi Neelaveni Kumari W/o Gopala Krishna, 22 years, C/SC Mala, Ramannapalem (V), Kamavarapukota (M) reported that the complainant’s sister named by Polimetla Sirisha, W/o Moshe, 20 years, SC Mala, Jogadigudem H/o Kamavarapukota (V)&(M) went to Ch.Pothepalli village, Dwaraka Tirumala mandal to see their mother along with her son Polimetla Manohar, S/o Gopala Krishna, 3 years, and her daughter Polimetla Mexica, D/o Gopala Krishna, 1 ½ years, on 04-01-2019. On 11-01-2019 at 3-30 PM told her mother that she is going to Jogadigudem and she would come to there for Sankranthi festival along with her husband. And left from there along with her childrens. After Sankranthi festival Sirisha’s fellow-daughter-in-law Grecemma phone called to the complainant’s aunt and asked about Sirisha, then her aunt replied that already she arrived there. -
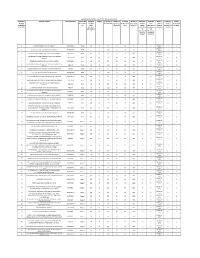
Not Applicable for IOC/HPC
APPOINTMENT OF RETAIL OUTLET DEALERSHIPS IN AP BY IOC Location Sl. Name Of Location Revenue District Type of RO Estimated Category Type of Site Minimum Minimum Minimum Estimated Estimated Mode of Fixed Fee / Security No. (Not (Regular/Rur monthly (CC/DC/CFS) Frontage of Depth of Site Area of site working fund selection Min bid Deposit ( Rs applicable al) Sales Site (in M) (in M) (in Sq. M.). capital required for (Draw of amount ( Rs in Lakhs) for IOC/HPC) Potential requirement developmen Lots/Bidding in Lakhs) (MS+HSD) in for t of ) Kls operation of infrastructur RO (Rs in e at RO (Rs Lakhs) in Lakhs ) DRAW OF 1 BUKKAPATNAM VILLAGE & MANDAL ANANTAPUR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 2 GOTLUR VILLAGE, DHARMAVARAM MANDAL ANANTAPUR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 3 VAYALPADU (NOT ON NH - SH), VAYALAPADU MANDAL CHITTOOR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS THONDAVADA VILLAGE (NOT ON NH/SH), CHANDRAGIRI DRAW OF 4 CHITTOOR Rural 48 SC CFS 20 20 400 0 0 0 2 MANDAL LOTS DRAW OF 5 DODDIPALLE (NOT ON NH/SH), PILERU MANDAL CHITTOOR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS NARAYANA NELLORE VILLAGE (NOT ON SH/NH) NANDALUR DRAW OF 6 KADAPA Rural 48 SC CFS 20 20 400 0 0 0 2 MANDAL LOTS DRAW OF 7 ARAKATAVEMULA NOT ON SH/NH , RAJUPALEM MANDAL KADAPA Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 8 GUTTURU VILLAGE, PENUKONDA MANDAL ANANTAPUR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 9 MADDALACHERUVU VILLAGE, KANAGANAPALLE MANDAL ANANTAPUR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 10 KALICHERLA (NOT ON NH/SH), PEDDAMANDYAM MANDAL CHITTOOR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS CHINNACHEPALLE, NOT ON SH/ NH, KAMALAPURAM DRAW OF 11 KADAPA Rural 48 SC CFS 20 20 400 0 0 0 2 MANDAL LOTS DRAW OF 12 GUDIPADU NOT ON SH/NH, DUVVUR MANDAL KADAPA Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS BUGGANIPALLE VILLAGE NOT ON NH/SH, BETHAMCHERLA DRAW OF 13 KURNOOL Rural 48 SC CFS 20 20 400 0 0 0 2 MANDAL LOTS DRAW OF 14 GOVINDPALLE VILLAGE NOT ON NH/SH, SIRVEL MANDAL KURNOOL Rural 48 ST CFS 20 20 400 0 0 0 2 LOTS DRAW OF 15 POLAKAL VILLAGE NOT ON NH/SH, C . -

Sri Vasavi Engineering College
SRI VASAVI ENGINEERING COLLEGE (Sponsored by Sri Vasavi Educational Society, Tadepalligudem) (Approved by AICTE, New Delhi & Accredited by NAAC with ‘A’ Grade) (Permanently affiliated to JNTUK & Recognized by UGC under section 2(f) & 12(B) Pedatadepalli, TADEPALLIGUDEM – 534 101. W.G.Dist. (A.P.) LIST OF CANDIDATES APPLIED FOR ADMISSION INTO UG PROGRAMMES (B.TECH. COURSES) UNDER CATEGORY ‘B’ (MANAGEMENT QUOTA) SEATS FOR THE ACADEMIC YEAR 2017-18 AS ON DATE 15-07-2017 S.No. Name of the Candidate Rank Details % of Marks in Branch Application Remarks and Address In JEE-Mains/ Qualifying Opted for Registration APEAMCET Examination Admn. In Date KOPPINEEDI NAVYA SATYA SRI NQ 91.2 1 D/o K.Venkata Rama Rao CSE 11-07-2017 Nil Attili Mandal & Post West Godavari Dist. MULLAPUDI POOJA D/o Mullapudi Bhima Raju 2 Lakkavaram Post 104212 86.6 CSE 11-07-2017 Nil Jangareddy Gudem Mandal West Godavari Dist. YADALAPALLI D P RAGHAVA S/o Yadlapalli Dharma Rao 3 82008 87.8 CSE 11-07-2017 Nil Ganapavaram Post & Mandal West Godavari Dist. YADAVALLI PURNA SAI S/o Yadavalli Rambabu 4 Pedavellamilli Post 86500 91.0 CSE 11-07-2017 Nil Ungutur Mandal West Godavari Dist. TALLAPRAGADA RAJA RAVI TEJA S/o T D N S S 5 SARVESWARARAO 64509 90.1 ECE 11-07-2017 Nil K N Road, TADEPALLIGUDEM – 534 101. West Godavari Dist. RAJAMAHENDRAVARAPU DANESWARA RAO S/o R.Srinivas 6 NQ 91.1 ECE 11-07-2017 Nil Chivatam Post Undrajavaram Mandal West Godavari Dist. BOYAPATI MURALI CHOUDARI 7 S/o Boyapati Venkateswara Rao 50437 96.2 ECE 11-07-2017 Nil Chintalapudi Mandal & Post West Godavari Dist. -

CSP Details for Basix Sub K I Transacations
Sr. CSP Location Name of BC-CSP Agent Adddress Contact Number Villages covered No. 1 Puppalapalle Chatla Sanjeev H no 2-10, Puppalapalle, Jakranpally, Nizamabad, AP -503003 94922 54471 Puppalapalle 2 Gaggupalle Miripala Mallaiah H no 1-49, Gaggupalli, Armoor, Nizamabad, Andhra Pradesh - 503224 88974 24849 Gaggupalle 3 Wandrikal Govindhu Arlappa H no 1-61, Wandrikal village, Gandhari Mandal, Nizamabad, Andhra Pradesh- 94927 29813 Wandrikal 503120 4 Brahmanapalle Elupula Bhaskar H no 1-12/1, Bhramanpalli village, Gandhari Mandal, Nizamabad , Andhra 77020 47793 Brahmanapalle Pradesh-503114 5 Gujjul Chitike Raju H no 1-98, Gujjal Village, Gandhari Mandal, Nizamabad, Andhra Pradesh- 94915 34542 Gujjul 503114 6 Durgam Gone Vishnu H no 1-17, Durgam Village, Gandhrari Mandal, Nizamabad Andhra Pradesh - 94936 62642 Durgam , Somaram 503114 7 Tipparam Yerram Bharati H no 1-81/1, Tipparam Village,Gandhari Mandal, Nizamabad District, Andhra 94905 08158 Tipparam Pradesh-503114 8 Tekrial Nangunuri Venkatesham H no 1-23, Tekiryal Village, Kamareddy Mandal, Nizamabad, Andhra Pradesh- 96184 91758 Tekrial 503111 9 Ravutla Sithap Naresh Kumar H no 5-61/1, Harijanawada, Ravutla village, Sirkonda Mandal, Nizamabad Dist, 99669 07301 Ravutla, Salampur AP-503165 10 Sikindrapur Barla Sanjeev H no 1-15/13, Jakranpally, Sikindrapur, Nizamabad, Telangana-503175 81438 12174 Sikindrapur, Madhapur 11 Kelojiwadi Allipuram Ranjith Kumar H no 2-74/A, Kalojiwadi village, Tadwai Mandal, Nizamabad District, 94942 61530 Kelojiwadi Telangana-503145 12 Manoharabad Chintakindi -
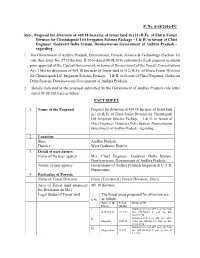
F. No. 8-38/2016-FC Sub.: Proposal for Diversion of 469.18 Hectares Of
F. No. 8-38/2016-FC Sub.: Proposal for diversion of 469.18 hectares of forest land in (12) R.Fs. of Eluru Forest Division for Chintalapudi Lift Irrigation Scheme Package – I & II, in favour of Chief Engineer, Godavari Delta System, Dowleswaram Government of Andhra Pradesh.– regarding. 1. The Government of Andhra Pradesh, Environment, Forests, Science & Technology (Section. II) vide their letter No. 3733/Section. II/2016 dated 09.08.2016 submitted a fresh proposal to obtain prior approval of the Central Government, in terms of the section-2 of the Forest (Conservation) Act, 1980 for diversion of 469.18 hectares of forest land in (12) R.Fs. of Eluru Forest Division for Chintalapudi Lift Irrigation Scheme Package – I & II, in favour of Chief Engineer, Godavari Delta System, Dowleswaram Government of Andhra Pradesh. 2. Details indicated in the proposal submitted by the Government of Andhra Pradesh vide letter dated 09.08.2016 are as below: FACT SHEET 1. Name of the Proposal Proposal for diversion of 469.18 hectares of forest land in (12) R.Fs. of Eluru Forest Division for Chintalapudi Lift Irrigation Scheme Package – I & II, in favour of Chief Engineer, Godavari Delta System, Dowleswaram Government of Andhra Pradesh.–regarding. 2. Location: State Andhra Pradesh. District West Godavari District. 3. Detail of user agency Name of the user agency M/s. Chief Engineer, Godavari Delta System, Dowleswaram, Government of Andhra Pradesh. Nature of user agency Government of Andhra Pradesh Irrigation & C.A.D. Department. 4. Particular of Forests Name of Forest Division Eluru (Territorial) Forest Division, Eluru. Area of Forest land proposed 469.18 Hectares. -

Farms Permitted for Culture SPF L. Vannamei
COASTAL AQUACULTURE AUTHORITY Ministry of Agriculture, Government of India Farms permitted for culture SPF L. vannamei Sl. Name of the Firm/Applicant & Address Total Water CAA Registration Location of the No for communication Farm Spread Number Farm Area Area (ha) (ha) 1. M/s.Onaway Industries Ltd. Bawaria Falia, Matruchaya Complex, Mendher Village, 100.00 59.00 GJ-II-2008 (0099) Navjivan Colony, Navsari District, Bilimora – 396321, Gujarat Gujarat 2. M/s.Siri Aqua Fams & Exports Pvt Ltd. Etadam, Payaka Falt No: 204, Sita Towers, ASR Nagar, Rao J.P. Road, Bhimavaram – 534 202, 71.30 63.15 AP-II-2009 (7733) Peta Mandal, West Godavari District, A.P Visakhapatnam District 3. Shri.V. Vasant Kumar Chollangi Village Chollangi Village, Tallarevu Mandal, 11.90 6.00 AP-II-2009 (8787) Tallarevu Mandal East Godavari District- 533001, Andhra Pradesh 4. Smt.V.Uma Devi Chollangi Village Chollangi Village, Tallarevu Mandal, 7.20 5.80 AP-II-2009 (8788) Tallarevu Mandal East Godavari district- 533001, Andhra Pradesh 5. M/s.Prathyusha Global Trade pvt. Ltd. Ravivaripalem Ravivaripalem Village, 28.50 21.00 AP-II-2009 (7749) Village, Tangutur(M) Tangutur Mandal Prakasam District, Andhra Pradesh 6. M/s.Devi Sea Foods Ltd. Gadepalem 9-14-8/1, C.B.M. Compound, Village, 40.00 25.00 AP-II-2009 (7742) Visakhapatnam – 530003, Kothapatnam Andhra Pradesh Mandal 7. M/s.Devi Fisheries Ltd. Gadepalem 7-8-20/1, Kasturiba Marg, Village, Near Ramakrishna Mission, 36.00 23.00 AP-II-2009 (7741) Kothapatnam Visakhapatnam – 530 003, Mandal Andhra Pradesh 8. M/s. United Aqua Farms, AP-II-2008 (3667, Kumaragiripatnam Godi Village, Allavaram Mandal, 6.60 5.00 3681 , 3682, 3666, Village, Allavaram East Godavari District – 533 217, 4070, 4061, 3671) Mandal, Andhra Pradesh 9. -

In the High Court of Karnataka at Bengaluru
IN THE HIGH COURT OF KARNATAKA AT BENGALURU DATED THIS THE 28 TH DAY OF APRIL 2016 BEFORE THE HON’BLE MR.JUSTICE S. ABDUL NAZEER WRIT PETITION NOS.25666-25714/2016 (EDN-RES) 1. PRASHANTH D.Ed COLLEGE ERNAGANAHALLI ROAD, OORGAUMPET, KOLAR GOLD FIELDS, BANGARPET TALUK, KOLAR DISTRICT. REPRESENTED BY ITS SECRETAY, SRI.N.R.VIJAYASHANKAR, AGED ABOUT 55 YEARS, SON OF SRI RAMAPPA, R/O OPPOSITE TO GENERAL HOSPITAL, ROBERTSONPET, KOLAR GOLD FIELDS-563122. BANGARAPET TALUK, KOLAR DISTRICT. 2. ANIGE SANTHA KUMARI D/O.ANIGE KOVVADAYYA, AGED:28 YAERS, JODEBODIGUDEM VILLAGE, DORAMAMIDI MANDAL, WEST GODIVARI, ANDHRA PRADESH. 3. BHOGI RAJU TATI S/O.BHIMARAJU, AGED:27 YEARS, WEST GODIVARI, ANDHRA PRADESH. 2 4. DURGAMMA TELLAM D/O.VENKATESWARA RAO, AGED:31 YEARS, GANGANNAGUDEM VILLAGE, JEELUGUMILLI MANDAL WEST GODAVARI DISTRICT, ANDHRA PRADESH. 5. BANDHAM BHASKARA RAO S/O KOVVADAYYA, AGED:29 YERS, WEST GODIVARI, ANDHRA PRADESH. 6. BALANNA KUTHANGI S/O.PEDAAPALAKONDA, AGED:31 YEARS, HUNKUMPETA MANDAL, ANDHRA PRADESH. 7. BHANU MADAKAM D/O DURGA RAO, AGED:27 YEARS, BUTTAYAGUDEM MANDAL, BUTTAYAGUDEM DISTRICT, WEST GODIVARI, ANDHRA PRADESH. 8. BHOJARAJU DIVYA BHARATHI D/O BHOJA RAJU RAMANA, AGED24 YEARS, JAMI MANDAL, VIZIANGARAM DISTRICT, ANDHRA PRADESH. 9. CHANDRAVATHI MADAKAM, D/O.GANGARAJU, AGED:28 YEARS, WEST GODIVARI, ANDHRA PRADESH. 10. CHIRRI KAMALA KUMAR D/O.CHIRRI MUTYALU, AGED:26 YEARS, WEST GODIVAI, ANDHRA PRADESH. 3 11. DURGA RAO KUNJA S/O.VENKAPPA, AGED:33 YEARS, JELLUGUMILLI M MANDAL, WEST GODIVARI, ANDHRA PRADESH. 12. GANAPTHI SOYAM S/O.RAJALU, AGED:31 YEARS, KANNAPURAM POST, WEST GODIVARI, ANDHRA PRADESH. 13. JALAGAM SEETA RAMA LAKSHMI D/O.JALAGAM PENTAYYA, AGED:22 YEARS, BUTTAYAGUDEM MANDAL, WEST GODIVRI, ANDHRA PRADESH. -

Missing Person - Period Wise Report (CIS) 26/02/2019 Page 1 of 50
Missing Person - Period Wise Report (CIS) 26/02/2019 Page 1 of 50 Crime No., U/S, PS, Name District 8/2019 for U/S Woman-Missing Person of the case of Kothapalli PS, Kurnool Dst, Andhra Pradesh Name Dol Sunitha Father Name Dol Pedda Naganna Gender Female Age 22 Age Missing Date 21-01-2019 Missing from Location Contact Phone 0 G Veerapuram,G Veerapuram,kothapalli, Kurnool, Andhra Contact Address Pradesh Languages Known Approx. Height 0.0 Hair Complexion Built ID Marks - Articles Found Mental Condition Date of FIR 21/01/2019 PS Phone - Brief Facts of the Case Occurred Prior to 21.01.2019 at about 12.00 hours and reported in the P.S. on 21.01.2019 at 12.00 hrs, in which the missing woman Dol Sunitha D/o D.Pedda Naganna age 22 years resident of G. Veerapuram village, Kothapalli mandal, now preparing for DSC exam at Pratibha Coaching centre in Kurnool. Complainant / Missing woman Father Dol Pedda Naganna s/o D.Ramanna age 48 years of same village. Phoned to missing women but she did not replay, later he sent his son to Kurnool for his daughter but she is not there since 01.12.2018 since then, she is missing and the complainant suspected that the missing woman eloped with someone. 26/02/2019 Page 2 of 50 Crime No., U/S, PS, Name District 23/2019 for U/S Man-Missing Person of the case of Nagaram PS, Guntur Dst, Andhra Pradesh Name Mannem Srikanth Father Name Venkateswararao Gender Male Age 26 Age Missing Date 21-01-2019 Missing from Location at complainant House Adda Contact Phone 0 Addankivaripalem village,Addankivaripalem Contact Address village,Nagaram, Guntur, Andhra Pradesh Languages Known Approx. -

A Survey on the Ethnomedicinal Practices of Konda Reddi Tribe from Polavaram Mandal, Andhra Pradesh, India
ISSN (e): 2250 – 3005 || Volume, 07 || Issue, 08|| August – 2017 || International Journal of Computational Engineering Research (IJCER) A Survey on the ethnomedicinal practices of Konda reddi tribe from Polavaram Mandal, Andhra Pradesh, India 1P. Prasanna Kumari*, 2Z.Vishnuvardhan 1. D.N.R.College, Bhimavaram – 534 201 2. Department of Botany & Microbiology Acharya Nagarjuna University, Nagarjuna Nagar-522510 AP, India Corresponding Author: P. Prasanna Kumari ABSTRACT The present study reports the ethnomedicinal practices of Konda reddi tribe from West Godavari district, Andhra Pradesh. The study area covers 10 out of 21 tribal villages of Polavaram Mandal where Konda reddis constitute the dominant community. The information was gathered through semi-structured interviews with the tribal practitioners and knowledgeable elders of the tribal villages. The present study has resulted in the documentation of 64 medicinal plant species belonging to 36 families and 57 genera. Altogether, 50 types of ailments have been reported to be cured by using these 64 plant species. Of the different plant parts, leaf was used in the majority of remedies 55(36.67%), followed by root 33(22%), stem bark 26(17.33%), Whole plant 11(7.33%), fruit 8(5.33%) and seed 5(3.33%). 16 plants of present study have been already known to be similarly used by the different tribes in different districts of Andhra Pradesh. The study thus emphasizes the need to make further pharmacological and photochemical investigations on these 16 plant species. Keywords: Phytomedicines, Traditional knowledge, Indigenous people, Primary healthcare, Bioactive compounds. ----------------------------------------------------------------------------------------------------------------------------- ---------- Date of Submission: 10-08-2017 Date of acceptance: 24-08-2017 ----------------------------------------------------------------------------------------------------------------------------- ---------- I. -

LIST of FARMS REGISTERED in EAST GODAVARI DISTRICT * Valid for 5 Years from the Date of Issue
LIST OF FARMS REGISTERED IN EAST GODAVARI DISTRICT * Valid for 5 Years from the Date of Issue. Address Farm Address S.No. Registration No. Name Father's / Husband's name Survey Number Issue date * Village / P.O. Mandal District Mandal Revenue Village Karri Venkat C/o D Divakara Reddy, 116/3; 114/5,7,11; 1 AP-II-2007(01621) Krishna Reddy Shri Venkat Reddy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 110/1 26.11.2007 Medapati Sura C/o D Divakara Reddy, 120/1A, 122/3 to 5; 2 AP-II-2007(01622) Reddy Shri Rama Reddy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 111/1 26.11.2007 Katta Veera C/o D Divakara Reddy, 3 AP-II-2007(01623) Raghavalu Shri Venkanna Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 122/2 26.11.2007 Boddupalli Appa C/o D Divakara Reddy, 4 AP-II-2007(01624) Rao Shri Appala Swamy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 112/1,4,5,6,2,8,10 26.11.2007 112/9, 111/4, 112/3, C/o D Divakara Reddy, 112/7, 111/2, 112/1, 5 AP-II-2007(01625) Bera Setha Ramulu Shri Suryanarayana Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 120/B; 122/1 26.11.2007 Palepu Venkat C/o D Divakara Reddy, 111/3, 111/5, 111/6, 6 AP-II-2007(01626) Ramana Shri Chinna Appala Swamy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 111/7 26.11.2007 Dwarampudi C/o D Divakara Reddy, 7 AP-II-2007(01627) Divakara Reddy Shri Appa Reddy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi -

Polavaram Mandal, Westgodavari District, Andhra Pradesh, India
ISSN : 2348-0033 (Online) ISSN : 2249-4944 (Print) IJEAR VOL . 6, ISSU E 2, SPL - 2, JULY - DE C 2016 Assesment of Ground Water Quality - Polavaram Mandal, Westgodavari District, Andhra Pradesh, India 1B.Ranjith Kumar, 2D.Chowdeswari, 3M.Anand Kumar, 4C.Ravi, 5R.Harinadha Babu, 6N V V S Prasad 1DST Project, Sir C R Reddy Educational Institutions, Eluru, AP, India 2,3Dept. of Chemistry, Sir C R Reddy college of Engineering, Eluru, AP, India 4Reader in Geology & Co-PI, Sir C R Reddy (A) College, Eluru, AP, India 5Dept. of Civil Engineering & Co-PI, Sir C R Reddy college of Engineering, Eluru, AP, India 6Reader in Chemistry &PI, Sir C R Reddy (A) College, Eluru, AP, India Abstract Water is an essential resource for life on the earth. Ground water is Fertilizers and pesticides are highly toxic and in countless cases, the major source of water for drinking, agricultural, and industrial quite mobile in the subsurface. Numerous compounds, however, desires. India is the largest user of groundwater in the world. In become quickly attached to fine-grained sediments, such as organic major part of our country ground water is the main source of matter and clay and silt particles. In many heavily fertilized areas, drinking water. In the present study polavaram mandal is chosen to the infiltration of nitrate, a decomposition product of ammonia assess the quality of drinking water. Samples were collected from fertilizer has adversely affected ground water. The consumption all the 12 villages in the Mandal and assessed for the suitability of nitrate rich water leads to a disease in infants known as “blue of human consumption.