Magnetic Resonance Imaging and Clinical Findings in Seminal Vesicle Pathologies ______
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CSI Study Guide-Female and Male Exams
Guide for Skill Station Female & Male Exams 2019 1. Overview Students will have the opportunity to perform female and male GU exams using both mannequins and standardized patients. The female standardized patient GU exam will include the external genitalia and pelvic exam, including use of a speculum; Male GU exam will include hernia and external genitalia/testicular examination. Practice session using mannequin will include evaluation of the prostate. Also refer to the Female and Male Exam – Factsheet 2019 for additional simulation lab session instructions 2. Goal of the Procedure Accurately perform female and male GU exams using proper techniques and logical sequence, while providing for patient comfort and modesty. 3. Reference(s) Jarvis, C. (2016). Physical Examination and Health Assessment. (7th ed.). Philadelphia: Elsevier. 4. Required Reading / Review Begin by reviewing the materials from 609a Health Assessment: a. Panoptos: Week 11 Male Genitourinary System: Anus, Rectum, Prostate: Male Genital Exam Week 12 Female Genital exam b. Jarvis, C. (2016). Physical Examination and Health Assessment. Pocket Guide (7th ed.). Philadelphia: Elsevier. Use above link, then use your UA Net ID Credentials to sign into the library, then click view full text, navigate to below chapters • Chapter 17 Male Genitourinary System pp 225-236; 12 pages • Chapter 18 Female Genitourinary System pp 237-252; 16 pages • Chapter 19 Anus, Rectum, and Prostate pp 253-260; 8 pages 5. Required Procedure Competencies Professionalism 1. Present/on time 2. Prepared (readings, etc.) 3. Engaged and participated 4. Respectful of others Communication skills 1. Obtain name and age of the patient and relationship of others if present 2. -

Table of Contents
viii Contents Chapter 1. Taking the Certification Examination . 1 General Suggestions for Preparing for the Exam About the Certification Exams Chapter 2. Developmental and Behavioral Sciences . 11 Mary Jo Gilmer, PhD, MBA, RN-BC, FAAN, and Paula Chiplis, PhD, RN, CPNP Psychosocial, Cognitive, and Ethical-Moral Development Behavior Modification Physical Development: Normal Growth Expectations and Developmental Milestones Family Concepts and Issues Family-Centered Care Cultural and Spiritual Diversity Chapter 3. Communication . 23 Mary Jo Gilmer, PhD, MBA, RN-BC, FAAN, and Karen Corlett, MSN, RN-BC, CPNP-AC/PC, PNP-BC Culturally Sensitive Communication Components of Therapeutic Communication Communication Barriers Modes of Communication Patient Confidentiality Written Communication in Nursing Practice Professional Communication Advocacy Chapter 4. The Nursing Process . 33 Clara J. Richardson, MSN, RN–BC Nursing Assessment Nursing Diagnosis and Treatment Chapter 5. Basic and Applied Sciences . 49 Mary Jo Gilmer, PhD, MBA, RN-BC, FAAN, and Paula Chiplis, PhD, RN, CPNP Trauma and Diseases Processes Common Genetic Disorders Common Childhood Diseases Traction Pharmacology Nutrition Chemistry Clinical Signs Associated With Isotonic Dehydration in Infants ix Chapter 6. Educational Principles and Strategies . 69 Mary Jo Gilmer, PhD, MBA, RN-BC, FAAN, and Karen Corlett, MSN, RN-BC, CPNP-AC/PC, PNP-BC Patient Education Chapter 7. Life Situations and Adaptive and Maladaptive Responses . 75 Mary Jo Gilmer, PhD, MBA, RN-BC, FAAN, and Karen Corlett, MSN, RN-BC, CPNP-AC/PC, PNP-BC Palliative Care End-of-Life Care Response to Crisis Chapter 8. Sensory Disorders . 87 Clara J. Richardson, MSN, RN–BC Developmental Characteristics of the Pediatric Sensory System Hearing Disorders Vision Disorders Conjunctivitis Otitis Media and Otitis Externa Retinoblastoma Trauma to the Eye Chapter 9. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Hyperplasia (Growth Factors
Adaptations Robbins Basic Pathology Robbins Basic Pathology Robbins Basic Pathology Coagulation Robbins Basic Pathology Robbins Basic Pathology Homeostasis • Maintenance of a steady state Adaptations • Reversible functional and structural responses to physiologic stress and some pathogenic stimuli • New altered “steady state” is achieved Adaptive responses • Hypertrophy • Altered demand (muscle . hyper = above, more activity) . trophe = nourishment, food • Altered stimulation • Hyperplasia (growth factors, . plastein = (v.) to form, to shape; hormones) (n.) growth, development • Altered nutrition • Dysplasia (including gas exchange) . dys = bad or disordered • Metaplasia . meta = change or beyond • Hypoplasia . hypo = below, less • Atrophy, Aplasia, Agenesis . a = without . nourishment, form, begining Robbins Basic Pathology Cell death, the end result of progressive cell injury, is one of the most crucial events in the evolution of disease in any tissue or organ. It results from diverse causes, including ischemia (reduced blood flow), infection, and toxins. Cell death is also a normal and essential process in embryogenesis, the development of organs, and the maintenance of homeostasis. Two principal pathways of cell death, necrosis and apoptosis. Nutrient deprivation triggers an adaptive cellular response called autophagy that may also culminate in cell death. Adaptations • Hypertrophy • Hyperplasia • Atrophy • Metaplasia HYPERTROPHY Hypertrophy refers to an increase in the size of cells, resulting in an increase in the size of the organ No new cells, just larger cells. The increased size of the cells is due to the synthesis of more structural components of the cells usually proteins. Cells capable of division may respond to stress by undergoing both hyperrtophy and hyperplasia Non-dividing cell increased tissue mass is due to hypertrophy. -

Reportable BD Tables Apr2019.Pdf
April 2019 Georgia Department of Public Health | Division of Health Protection | Maternal and Child Health Epidemiology Unit Reportable Birth Defects with ICD-10-CM Codes Reportable Birth Defects in Georgia with ICD-10-CM Diagnosis Codes Table D.1 Brain Malformations and Neural Tube Defects ICD-10-CM Diagnosis Codes Birth Defect ICD-10-CM 1. Brain Malformations and Neural Tube Defects Q00-Q05, Q07 Anencephaly Q00.0 Craniorachischisis Q00.1 Iniencephaly Q00.2 Frontal encephalocele Q01.0 Nasofrontal encephalocele Q01.1 Occipital encephalocele Q01.2 Encephalocele of other sites Q01.8 Encephalocele, unspecified Q01.9 Microcephaly Q02 Malformations of aqueduct of Sylvius Q03.0 Atresia of foramina of Magendie and Luschka (including Dandy-Walker) Q03.1 Other congenital hydrocephalus (including obstructive hydrocephaly) Q03.8 Congenital hydrocephalus, unspecified Q03.9 Congenital malformations of corpus callosum Q04.0 Arhinencephaly Q04.1 Holoprosencephaly Q04.2 Other reduction deformities of brain Q04.3 Septo-optic dysplasia of brain Q04.4 Congenital cerebral cyst (porencephaly, schizencephaly) Q04.6 Other specified congenital malformations of brain (including ventriculomegaly) Q04.8 Congenital malformation of brain, unspecified Q04.9 Cervical spina bifida with hydrocephalus Q05.0 Thoracic spina bifida with hydrocephalus Q05.1 Lumbar spina bifida with hydrocephalus Q05.2 Sacral spina bifida with hydrocephalus Q05.3 Unspecified spina bifida with hydrocephalus Q05.4 Cervical spina bifida without hydrocephalus Q05.5 Thoracic spina bifida without -

The Genitourinary System I
U.S. ARMY MEDICAL DEPARTMENT CENTER AND SCHOOL FORT SAM HOUSTON, TEXAS 78234-6100 THE GENITOURINARY SYSTEM I SUBCOURSE MD0579 EDITION 100 DEVELOPMENT This subcourse is approved for resident and correspondence course instruction. It reflects the current thought of the Academy of Health Sciences and conforms to printed Department of the Army doctrine as closely as currently possible. Development and progress render such doctrine continuously subject to change. ADMINISTRATION Students who desire credit hours for this correspondence subcourse must enroll in the subcourse. Application for enrollment should be made at the Internet website: http://www.atrrs.army.mil. You can access the course catalog in the upper right corner. Enter School Code 555 for medical correspondence courses. Copy down the course number and title. To apply for enrollment, return to the main ATRRS screen and scroll down the right side for ATRRS Channels. Click on SELF DEVELOPMENT to open the application; then follow the on-screen instructions. For comments or questions regarding enrollment, student records, or examination shipments, contact the Nonresident Instruction Branch at DSN 471-5877, commercial (210) 221-5877, toll-free 1-800-344-2380; fax: 210-221-4012 or DSN 471-4012, e-mail [email protected], or write to: NONRESIDENT INSTRUCTION BRANCH AMEDDC&S ATTN: MCCS-HSN 2105 11TH STREET SUITE 4191 FORT SAM HOUSTON TX 78234-5064 Be sure your social security number is on all correspondence sent to the Academy of Health Sciences. CLARIFICATION OF TERMINOLOGY When used in this publication, words such as "he," "him," "his," and "men" 'are intended to include both the masculine and feminine genders, unless specifically stated otherwise or when obvious in context. -

Medical Terminology
MEDICAL TERMINOLOGY TRI-COUNTY REGIONAL OCCUPATIONAL PROGRAM CDE COURSE 2268 CBEDS #4298 Description: This course is an introduction to medical terminology for those preparing for a health or business career such as nursing, medical secretary, ward secretary, emergency medical technician, respiratory therapist, or any other field requiring a medical vocabulary. This course articulates with the Yuba College 2+2 program, and is designed to meet the prerequisites for our ROP Vocational Nursing class and is one of the requirements for the Medical Office Services (MA) and Health Care Information Services courses. Performance Objectives: Upon completion of the Medical Terminology course, the student will understand: 1. The basic elements of a medical word. 2. Suffixes related surgical, diagnostic, symptomatic medical words. 3. Suffixes relative to medical words including adjectives, nouns, diminutives, singular and plural words. 4. Prefixes of medical words. 5. Medical words that define the human body in relation to disease processes, the four body planes, body cavities and organs, abdominopelvic regions, and demonstrating understanding of body imaging by defining terms associated with radiology, computed tomography, MRI and ultrasounds. 6. Medical words that identify the functions and body parts associated with the integumentary system. 7. Medical words that describe and define the gastrointestinal system. 8. Medical words that define and describe the respiratory system. 9. Medical words that define and describe the cardiovascular system. 10. Medical words that define and describe blood and lymphatic systems. 11. Medical words that define and describe the musculoskeletal system. 12. Medical words that define and describe the genitourinary system. 13. Medical words that define and describe the female reproductive system. -
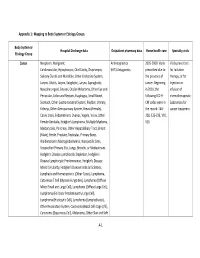
A-1 Appendix 1
Appendix 1: Mapping to Body System or Etiology Groups Body System or Hospital Discharge data Outpatient pharmacy data Home health care Specialty visits Etiology Group Cancer Neoplasm, Malignant: Antineoplastics 2005 -2009: Visits Visits prescribed Cardiovascular, Hypopharynx, Oral Cavity, Oropharynx, 5HT3 Antagonists prescribed due to for radiation Salivary Glands and Mandible, Other Endocrine System, the presence of therapy, or for Larynx, Glottis, Larynx, Subglottic, Larynx, Supraglottic, cancer. Beginning Injection or Nasopharyngeal, Sinuses, Ocular Melanoma, Other Eye and in 2010, the infusion of Periocular, Colon and Rectum, Esophagus, Small Bowel, following ICD-9- chemotherapeutic Stomach, Other Gastrointestinal System, Bladder, Urinary, CM codes were in Substances for Kidneys, Other Genitourinary System, Breast (Female), the record: 140- cancer treatment Cervix Uteri, Endometrium, Ovaries, Vagina, Vulva, Other 208, 235-239, V10, Female Genitalia, Hodgkin's Lymphoma, Multiple Myeloma, V16 Mastocytosis, Pancreas, Other Hepatobiliary Tract, Breast (Male), Penile, Prostate, Testicular, Primary Bone, Waldenstrom's Macroglobulinemia, Nonspecific Sites, Unspecified Primary Site, Lungs, Bronchi, or Mediastinum, Hodgkin's Disease Lymphocytic Depletion, Hodgkin's Disease Lymphocytic Predominance, Hodgkin's Disease Mixed Cellularity, Hodgkin's Disease Nodular Sclerosis, Lymphatic and Hematopoietic (Other Types), Lymphoma, Cutaneous T Cell (Mycosis Fungoides), Lymphoma (Diffuse Mixed Small and Large Cell), Lymphoma (Diffuse Large Cell), Lymphoma -

Chapter 1 Cellular Reaction to Injury 3
Schneider_CH01-001-016.qxd 5/1/08 10:52 AM Page 1 chapter Cellular Reaction 1 to Injury I. ADAPTATION TO ENVIRONMENTAL STRESS A. Hypertrophy 1. Hypertrophy is an increase in the size of an organ or tissue due to an increase in the size of cells. 2. Other characteristics include an increase in protein synthesis and an increase in the size or number of intracellular organelles. 3. A cellular adaptation to increased workload results in hypertrophy, as exemplified by the increase in skeletal muscle mass associated with exercise and the enlargement of the left ventricle in hypertensive heart disease. B. Hyperplasia 1. Hyperplasia is an increase in the size of an organ or tissue caused by an increase in the number of cells. 2. It is exemplified by glandular proliferation in the breast during pregnancy. 3. In some cases, hyperplasia occurs together with hypertrophy. During pregnancy, uterine enlargement is caused by both hypertrophy and hyperplasia of the smooth muscle cells in the uterus. C. Aplasia 1. Aplasia is a failure of cell production. 2. During fetal development, aplasia results in agenesis, or absence of an organ due to failure of production. 3. Later in life, it can be caused by permanent loss of precursor cells in proliferative tissues, such as the bone marrow. D. Hypoplasia 1. Hypoplasia is a decrease in cell production that is less extreme than in aplasia. 2. It is seen in the partial lack of growth and maturation of gonadal structures in Turner syndrome and Klinefelter syndrome. E. Atrophy 1. Atrophy is a decrease in the size of an organ or tissue and results from a decrease in the mass of preexisting cells (Figure 1-1). -

CELL INJURY MEDICAL (Lecture 1)
CELL INJURY MEDICAL (lecture 1) Sufia Husain Assistant Prof & Consultant College of Medicine, KSU, Riyadh. September 2015 Objectives for Cell Injury Chapter (3 lectures) The students should: A. Understand the concept of cells and tissue adaptation to environmental stress including the meaning of hypertrophy, hyperplasia, aplasia, atrophy, hypoplasia and metaplasia with their clinical manifestations. B. Is aware of the concept of hypoxic cell injury and its major causes. C. Understand the definitions and mechanisms of free radical injury. D. Knows the definition of apoptosis, tissue necrosis and its various types with clinical examples. E. Able to differentiate between necrosis and apoptosis. F. Understand the causes of and pathologic changes occurring in fatty change (steatosis), accumulations of exogenous and endogenous pigments (carbon, silica, iron, melanin, bilirubin and lipofuscin). G. Understand the causes of and differences between dystrophic and metastatic calcifications. Lecture 1 outline . Adaptation to environmental stress: hypertrophy, hyperplasia, aplasia, hypoplasia, atrophy, squamous metaplasia, osseous metaplasia and myeloid metaplasia. Hypoxic cell injury and its causes (ischaemia, anaemia, carbon monoxide poisoning, decreased perfusion of tissues by oxygen, carrying blood and poor oxygenation of blood). Free radical injury: definition of free radicals, mechanisms that generate free radicals, mechanisms that degrade free radicals. Reversible and irreversible cell injury ADAPTATION TO ENVIRONMENTAL STRESS Adaptation to environmental stress . Cells are constantly adjusting their structure and function to accommodate changing demands i.e. they adapt within physiological limits. As cells encounter physiologic stresses or pathologic stimuli, they can undergo adaptation. The principal adaptive responses are . hypertrophy, . hyperplasia, . atrophy, . metaplasia. (NOTE: If the adaptive capability is exceeded or if the external stress is harmful, cell injury develops. -
![The Genitourinary System (And] Instructor's Guide: the Genitourinary System](https://docslib.b-cdn.net/cover/6525/the-genitourinary-system-and-instructors-guide-the-genitourinary-system-1156525.webp)
The Genitourinary System (And] Instructor's Guide: the Genitourinary System
DOCUMENT RESUME ED 213 968 CE 031 777 TITLE The Genitourinary System (and] Instructor's Guide: The Genitourinary System. Health Occupations Education Module: Instructional Materials in Anatomy and Physiology for Pennsylvania Health Occupations Programs. INSTITUTION National Evaluation Systems, Inc., Amherst, Mass. SPONS AGENCY Pennsylvania State Dept. of Education, Harrisburg. Bureau of Vocational and Technical Education. PUB DATE atm 80 NOTE 33p.; For related documents see listing in note of CE 031 758. EDRS PRICE MF01/PCO2 Plus Postage. DESCRIPTORS ' *Allied Health Occupations Education; *Anatomy; Behaviorpl Objectives; *Individualized Instruction; *Learning Activities; Learning Modules; *Males; Medical Vocabulary; *Physiology; Postsecondary Education; Pretests Posttests; Programed Instructional Materials; Secondary Education; Self Evaluation (Individuals); Teaching Methods IDENTIFIERS *Genitourinary System; Pennsylvania ABSTRACT This module on the genitourinary system is one of 17 modules designed for individualized instruction in health occupations education programs at both the secondary and postsecondary levels. It is part of an eight-unit miniseries on anatomy, and physiology within the series of 17 modules. Following a preface which explains to the student how to use the module, the unit consists of a pretest with answers, three sections (information sheets) with their objectives (e.g., identify and describe the location and anatomical structure of the kidneys), optional activities (e.g., research the process of _____dialysi-sby an artificial kidney machine), and posttests, and a glossary of terms. Topics covered in the unit are introduction to the genitourinary system, the urinary system, and the male reproductive system. An accompanying instructor's guide Contains suggestions for using the module and answers to the posttest. (KC) *********************************************************************** * Reproductions supplied by EDRS are the best that can be made * * from the original document. -

Association of Syndactyly, Ectodermal Dysplasia, and Cleft Lip and Palate: Report of Two Sibs from Turkey
J Med Genet: first published as 10.1136/jmg.25.1.37 on 1 January 1988. Downloaded from Journal of Medical Genetics 1988, 25, 37-40 Association of syndactyly, ectodermal dysplasia, and cleft lip and palate: report of two sibs from Turkey GONUL O(UR AND MEMNUNE YUKSEL From the Institute of Child Health, University of Istanbul, (7apa/lIstanbul, Turkey. SUMMARY Two Turkish sibs, products of a second cousin marriage, with tetramelic syndactyly, ectodermal dysplasia, cleft lip and palate, renal anomalies, and mental retardation are reported. Similarities between these two brothers and previously reported cases and their mode of transmission are discussed. In 1961, Rosselli and Gulienettil reported four was said to have a cleft lip deformity. Unfortunately patients with hypohidrosis, hypotrichosis, micro- no more details were available. The family's first dontia, dystrophic nails, cleft lip and palate, deform- offspring was aborted in early pregnancy. The ities of the extremities, and malformations of the second, a healthy boy, is 11 years old. The third genitourinary system. Syndactyly was the predomi- pregnancy ended as a stillbirth but had no congenital nant digital deformity. Phenotypically normal par- malformations. The fourth and fifth children are our copyright. ents who were first cousins suggested an autosomal probands. recessive mode of inheritance in two of their cases who were sibs. In 1970, a very similar clinical CASE 1 condition was described as the EEC syndrome The older son (IV.4, fig 1) was born on 24.11.77, (ectrodactyly-ectodermal dysplasia-cleft lip/palate) weighing 2300 g, after a normal 32 week gestation. by Rudiger et a12 and Freire-Maia3 almost simul- No perinatal complications were observed.