Gasterophilus Pecorum (Diptera: Gasterophilidae) Population In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolutionary History of Stomach Bot Flies in the Light of Mitogenomics
Evolutionary history of stomach bot flies in the light of mitogenomics Yan, Liping; Pape, Thomas; Elgar, Mark A.; Gao, Yunyun; Zhang, Dong Published in: Systematic Entomology DOI: 10.1111/syen.12356 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Yan, L., Pape, T., Elgar, M. A., Gao, Y., & Zhang, D. (2019). Evolutionary history of stomach bot flies in the light of mitogenomics. Systematic Entomology, 44(4), 797-809. https://doi.org/10.1111/syen.12356 Download date: 28. Sep. 2021 Systematic Entomology (2019), 44, 797–809 DOI: 10.1111/syen.12356 Evolutionary history of stomach bot flies in the light of mitogenomics LIPING YAN1, THOMAS PAPE2 , MARK A. ELGAR3, YUNYUN GAO1 andDONG ZHANG1 1School of Nature Conservation, Beijing Forestry University, Beijing, China, 2Natural History Museum of Denmark, University of Copenhagen, Copenhagen, Denmark and 3School of BioSciences, University of Melbourne, Melbourne, Australia Abstract. Stomach bot flies (Calyptratae: Oestridae, Gasterophilinae) are obligate endoparasitoids of Proboscidea (i.e. elephants), Rhinocerotidae (i.e. rhinos) and Equidae (i.e. horses and zebras, etc.), with their larvae developing in the digestive tract of hosts with very strong host specificity. They represent an extremely unusual diver- sity among dipteran, or even insect parasites in general, and therefore provide sig- nificant insights into the evolution of parasitism. The phylogeny of stomach botflies was reconstructed -

Pest Management News
Pest Management News Dr. John D. Hopkins, Associate Professor and Extension Entomologist – Coeditor Dr. Kelly M. Loftin, Professor and Extension Entomologist – Coeditor Contributors Dr. Becky McPeake, Professor and Wildlife Extension Specialist Sherrie E. Smith, Plant Pathology Instructor, Plant Health Clinic Diagnostician Letter #6 October 31, 2017 ________________________________________________________________________________ Stopping Occasional Arthropod Invaders John D. Hopkins When the weather begins to change in the fall and things get cooler, arthropod pests like the multi-colored Asian lady beetle, the boxelder bug, crickets, various stinkbugs, or spiders are just some of the pest problems that homeowners may have to deal with. The first thing most people think of when trying to prevent a pest problem is WHAT INSECTICIDE DO I SPRAY? However, there are other measures that should be taken that will help prevent these pests from entering your home and may even eliminate the need for an insecticide application. Pest proofing your home is the BEST way to prevent unwanted invaders at this time or any other time of year. Your goal is to prevent pest entry and eliminate conditions that are conducive to pest infestation. Here are the ABC’s of pest proofing your home: A. Ensure that screens on doors and windows are properly installed and maintained. If you don't have screen doors on your home, install them. Any damaged screens should be repaired or replaced. Fine mesh screening will prevent all but the tiniest insects from entering your home. B. Doors should seal properly. If air can pass through or light can be seen through cracks around doors then insects or spiders can get in. -
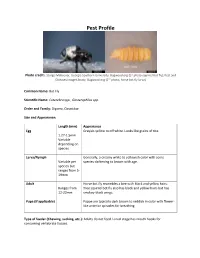
Bot Fly); Pest and Diseases Image Library, Bugwood.Org (2Nd Photo, Horse Bot Fly Larva)
Pest Profile Photo credit: Sturgis McKeever, Georgia Southern University, Bugwood.org (1st photo-squirrel bot fly); Pest and Diseases Image Library, Bugwood.org (2nd photo, horse bot fly larva) Common Name: Bot Fly Scientific Name: Cuterebra spp., Gasterophilus spp. Order and Family: Diptera, Oestridae Size and Appearance: Length (mm) Appearance Egg Grayish-yellow to off white. Looks like grains of rice. 1.27-1.5mm Variable depending on species Larva/Nymph Generally, a creamy white to yellowish color with some Variable per species darkening to brown with age. species but ranges from 5- 19mm Adult Horse bot fly resembles a bee with black and yellow hairs. Ranges from Tree squirrel bot fly also has black and yellow hairs but has 12-22mm smokey-black wings. Pupa (if applicable) Pupae are typically dark brown to reddish in color with flower- like anterior spiracles for breathing. Type of feeder (Chewing, sucking, etc.): Adults do not feed. Larval stage has mouth hooks for consuming vertebrate tissues. Hosts: The larval stages are obligate parasites that feed on the living tissues of their mammalian hosts. Horse bot flies tend to target horses but will also invade other equine species such as donkeys and mules. Tree squirrel bot flies feed on eastern gray squirrels, fox squirrels, and chipmunks. Description of Damage (larvae and adults): Tree squirrel bots are generally of no consequence other than the possibility of accidental myiasis in a cat or dog. Horse bot flies can cause irritation and stress in horses they infect. When the larvae invade the gastrointestinal tract, severe complications can arise such as colic, blockages, and other ulcers or ruptures. -

Light and Electron Microscopy Studies of the Midgut and Salivary Glands of Second and Third Instars of the Horse Stomach Bot, Gasterophilus Intestinalis
Published in Medical and Veterinary Entomology 24, issue 3, 236-249, 2010 1 which should be used for any reference to this work Light and electron microscopy studies of the midgut and salivary glands of second and third instars of the horse stomach bot, Gasterophilus intestinalis L. ROELFSTRA1, M. VLIMANT1, B. BETSCHART1, K. PFISTER2 and P.-A. D I E H L1 1Department of Zoology, Institute of Biology, University of Neuchatˆ el, Neuchatˆ el, Switzerland 2Department of Veterinary Sciences, Institute of Tropical Medicine and Parasitology, Ludwig Maximilians University, Munich, Germany Abstract. A morphological study of the midgut and salivary glands of second and third instars of Gasterophilus intestinalis (De Geer) (Diptera: Oestridae) was conducted by light, scanning and transmission electron microscopy. The midgut is anteriorly delimited by a proventriculus, without caeca, and is composed of posterior foregut and anterior midgut tissue from which a double-layered peritrophic matrix is produced. The midgut can be divided into anterior, median and posterior regions on the basis of the structural and physiological variations of the columnar cells which occur along its length. Two other types of cell were identified: regenerative cells scattered throughout the columnar cells, and, more rarely, endocrine cells of two structural types (closed and open). Different secretion mechanisms (merocrine, apocrine and microapocrine) occur along the midgut epithelium. Abundant microorganisms are observed in the endoperitrophic space of the anterior midgut. The origin and nature of these microorganisms remain unknown. No structural differences are observed between the second and third instar midguts. The salivary glands of G. intestinalis second and third instars consist of a pair of elongated tubular structures connected to efferent ducts which unite to form a single deferent duct linked dorsally to the pharynx. -

Arthropod Parasites in Domestic Animals
ARTHROPOD PARASITES IN DOMESTIC ANIMALS Abbreviations KINGDOM PHYLUM CLASS ORDER CODE Metazoa Arthropoda Insecta Siphonaptera INS:Sip Mallophaga INS:Mal Anoplura INS:Ano Diptera INS:Dip Arachnida Ixodida ARA:Ixo Mesostigmata ARA:Mes Prostigmata ARA:Pro Astigmata ARA:Ast Crustacea Pentastomata CRU:Pen References Ashford, R.W. & Crewe, W. 2003. The parasites of Homo sapiens: an annotated checklist of the protozoa, helminths and arthropods for which we are home. Taylor & Francis. Taylor, M.A., Coop, R.L. & Wall, R.L. 2007. Veterinary Parasitology. 3rd edition, Blackwell Pub. HOST-PARASITE CHECKLIST Class: MAMMALIA [mammals] Subclass: EUTHERIA [placental mammals] Order: PRIMATES [prosimians and simians] Suborder: SIMIAE [monkeys, apes, man] Family: HOMINIDAE [man] Homo sapiens Linnaeus, 1758 [man] ARA:Ast Sarcoptes bovis, ectoparasite (‘milker’s itch’)(mange mite) ARA:Ast Sarcoptes equi, ectoparasite (‘cavalryman’s itch’)(mange mite) ARA:Ast Sarcoptes scabiei, skin (mange mite) ARA:Ixo Ixodes cornuatus, ectoparasite (scrub tick) ARA:Ixo Ixodes holocyclus, ectoparasite (scrub tick, paralysis tick) ARA:Ixo Ornithodoros gurneyi, ectoparasite (kangaroo tick) ARA:Pro Cheyletiella blakei, ectoparasite (mite) ARA:Pro Cheyletiella parasitivorax, ectoparasite (rabbit fur mite) ARA:Pro Demodex brevis, sebacceous glands (mange mite) ARA:Pro Demodex folliculorum, hair follicles (mange mite) ARA:Pro Trombicula sarcina, ectoparasite (black soil itch mite) INS:Ano Pediculus capitis, ectoparasite (head louse) INS:Ano Pediculus humanus, ectoparasite (body -
Taxonomic Review Of
A peer-reviewed open-access journal ZooKeys 891: 119–156 (2019) Taxonomic review of Gasterophilus 119 doi: 10.3897/zookeys.891.38560 CATALOGUE http://zookeys.pensoft.net Launched to accelerate biodiversity research Taxonomic review of Gasterophilus (Oestridae, Gasterophilinae) of the world, with updated nomenclature, keys, biological notes, and distributions Xin-Yu Li1,2, Thomas Pape2, Dong Zhang1 1 School of Ecology and Nature Conservation, Beijing Forestry University, Qinghua east road 35, Beijing 10083, China 2 Natural History Museum of Denmark, University of Copenhagen, Universitetsparken 15, Copenhagen, Denmark Corresponding author: Dong Zhang ([email protected]) Academic editor: R. Meier | Received 6 August 2019 | Accepted 22 October 2019 | Published 21 November 2019 http://zoobank.org/84BE68FC-AA9D-4357-9DA0-C81EEBA95E13 Citation: Li X-Y, Pape T, Zhang D (2019) Taxonomic review of Gasterophilus (Oestridae, Gasterophilinae) of the world, with updated nomenclature, keys, biological notes, and distributions. ZooKeys 891: 119–156. https://doi. org/10.3897/zookeys.891.38560 Abstract A taxonomic review of Gasterophilus is presented, with nine valid species, 51 synonyms and misspellings for the genus and the species, updated diagnoses, worldwide distributions, and a summary of biological information for all species. Identification keys for adults and eggs are elaborated, based on a series of new diagnostic features and supported by high resolution photographs for adults. The genus is shown to have its highest species richness in China and South Africa, with seven species recorded, followed by Mongolia, Senegal, and Ukraine, with six species recorded. Keywords biology, distribution, horse stomach bot fly, identification, nomenclature, taxonomy Introduction The oestrids or bot flies (Oestridae) are known as obligate parasites of mammals in their larval stage. -

Ultrastructure of Adult Gasterophilus Intestinalis (Diptera: Gasterophilidae) and Its Puparium
International Journal of Tropical Insect Science https://doi.org/10.1007/s42690-019-00084-9 ORIGINAL RESEARCH ARTICLE Ultrastructure of adult Gasterophilus intestinalis (Diptera: Gasterophilidae) and its puparium Marwa M. Attia1 & Nagla M.K. Salaeh2 Received: 12 June 2019 /Accepted: 2 December 2019 # African Association of Insect Scientists 2019 Abstract This research was conducted to identify the most common equine stomach bot fly by using light and scanning electron microscopy. Third instar larvae (n = 200) of Gasterophilus intestinalis were collected from stomach of slaughtered donkeys (Equus asinus) at Giza Zoo abattoir, Egypt, in July 2017 (the donkeys chosen were from Giza; Egypt). One hundred only were full mature 3rd instar larvae (the mature 3rd instar was active and has brown bands on its dorsal surface) which were incubated at 32 °C and 80–85% Relative Humidity (RH), for the development of adult. The following pupal parameters were recorded: prepupal period, number of pupated larvae, pupal period, and number of emerged adult and determination of adult sex ratio. Morphological description of pupae, puparium and adults were provided using light and scanning electron microscopy (SEM). The length of prepupal duration was five days and about 90% of the collected G. intestinalis larvae successfully pupated with pupal duration lasting for 21–25 days prior to emergence of the adult stage. Adults sorted according to sex show a female to male ratio of 8:1 (i.e. 80 females to 10 males). The pupae of G. intestinalis were brown to black in color. The adult head, thorax, abdomen, legs and wings of male and female were morphologically described using light and SEM. -

Aus Dem Institut Für Parasitologie Und Tropenveterinärmedizin Des Fachbereichs Veterinärmedizin Der Freien Universität Berlin
Aus dem Institut für Parasitologie und Tropenveterinärmedizin des Fachbereichs Veterinärmedizin der Freien Universität Berlin Entwicklung der Arachno-Entomologie am Wissenschaftsstandort Berlin aus veterinärmedizinischer Sicht - von den Anfängen bis in die Gegenwart Inaugural-Dissertation zur Erlangung des Grades eines Doktors der Veterinärmedizin an der Freien Universität Berlin vorgelegt von Till Malte Robl Tierarzt aus Berlin Berlin 2008 Journal-Nr.: 3198 Gedruckt mit Genehmigung des Fachbereichs Veterinärmedizin der Freien Universität Berlin Dekan: Univ.-Prof. Dr. L. Brunnberg Erster Gutachter: Univ.-Prof. em. Dr. Dr. h.c. Dr. h.c. Th. Hiepe Zweiter Gutachter: Univ.-Prof. Dr. E. Schein Dritter Gutachter: Univ.-Prof. Dr. J. Luy Deskriptoren (nach CAB-Thesaurus): Arachnida, veterinary entomology, research, bibliographies, veterinary schools, museums, Germany, Berlin, veterinary history Tag der Promotion: 20.05.2008 Bibliografische Information der Deutschen Nationalbibliothek Die Deutsche Nationalbibliothek verzeichnet diese Publikation in der Deutschen Nationalbibliografie; detaillierte bibliografische Daten sind im Internet über <http://dnb.ddb.de> abrufbar. ISBN-13: 978-3-86664-416-8 Zugl.: Berlin, Freie Univ., Diss., 2008 D188 Dieses Werk ist urheberrechtlich geschützt. Alle Rechte, auch die der Übersetzung, des Nachdruckes und der Vervielfältigung des Buches, oder Teilen daraus, vorbehalten. Kein Teil des Werkes darf ohne schriftliche Genehmigung des Verlages in irgendeiner Form reproduziert oder unter Verwendung elektronischer Systeme verar- beitet, vervielfältigt oder verbreitet werden. Die Wiedergabe von Gebrauchsnamen, Warenbezeichnungen, usw. in diesem Werk berechtigt auch ohne besondere Kennzeichnung nicht zu der Annahme, dass solche Namen im Sinne der Warenzeichen- und Markenschutz-Gesetzgebung als frei zu betrachten wären und daher von jedermann benutzt werden dürfen. This document is protected by copyright law. -

Presence of Gasterophilus Species in Horses in Van Region
YYU Veteriner Fakultesi Dergisi, 2010, 21 (2), 87 - 90 ORIGINAL ARTICLE ISSN: 1017-8422; e-ISSN: 1308-3651 Presence of Gasterophilus Species in Horses in Van Region Nalan ÖZDAL1 Kamile BİÇEK1 Özlem ORUNÇ2 Serdar DEĞER1 1 University of Yuzuncu Yil, Faculty of Veterinary Medicine, Parasitology Dept., Van, Turkey 2 University of Yuzuncu Yil, Faculty of Medicine, Van, Turkey Received: 30.03.2010 Accepted: 13.04.2010 SUMMARY Ten horses aged 3-4 from rural Van region in the Eastern border of Turkey were examined post- mortem for the presence of Gasterophilus larvae from December 2008 to March 2009. Stomachs and intestines were removed according to the suitable necropsy techniques and checked for Gasterophilus species. Three horses were infected by larvae of Gasterophilus spp. and one second stage larvae (L2) and 265 third stage larvae (L3) collected from infested horses. Three species of Gasterophilus were identified with the following total larvae number and rate, respectively: Gasterophilus nasalis (182 / 68.42%), Gasterophilus intestinalis (76 / 28.57%), Gasterophilus inermis (8 / 3%). Key Words Gasterophilus, Horse, Van Van Yöresindeki Atlarda Tespit Edilen Gasterophilus Türleri ÖZET Türkiye’nin doğu sınırındaki Van ilinin kırsal kesiminde yaşayan 3-4 yaşlarındaki on at Aralık 2008 – Mart 2009 tarihleri arasında Gasterophilus türlerinin varlığı yönünde post mortem incelenmiştir. Nekropsi tekniğine uygun olarak mide ve bağırsaklar çıkarılmış ve Gasterophilus türlerinin varlığı araştırılmıştır. Üç at Gasterophilus larvalarıyla enfeste bulunmuş ve bir adet ikinci dönem larva, 265 adet üçüncü dönem larva toplanmıştır. Çalışmada üç Gasterophilus türü teşhis edilmiş ve türlere göre toplam larva sayıları ve oranları Gasterophilus nasalis (182 / %68.42), Gasterophilus intestinalis (76 / %28.57), Gasterophilus inermis (8 / %3) olarak belirlenmiştir. -

Exposure to Gasterophilus Spp. in Horses in NW Spain by ELISA
Journal of Entomology and Zoology Studies 2016; 4(5): 621-624 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Exposure to Gasterophilus spp. in horses in NW JEZS 2016; 4(5): 621-624 © 2016 JEZS Spain by ELISA Received: 29-07-2016 Accepted: 30-08-2016 Silvia Miguélez, Ana M Araújo, Iván Francisco, José Suarez, Rita Silvia Miguélez Equine Diseases Study Group Sánchez-Andrade, Adolfo Paz-Silva and María Sol Arias (Epidemiology, Parasitology and Zoonoses), Animal Pathology Department, Veterinary Faculty, Abstract Santiago de Compostela University, Gasterophilosis is a myiasis related with gastric ulcers in equids and even colics. A serological survey to Lugo, Spain assess the presence of IgG antibodies against Gasterophilus spp. in horses from an oceanic climate area was conducted. A total of 672 blood samples were analyzed by an enzyme-linked immunosorbent assay Ana M Araújo Equine Diseases Study Group (ELISA) and excretory/secretory antigens from G. intestinalis second-instar larvae. Results were (Epidemiology, Parasitology and analyzed according to intrinsic and extrinsic factors. Sixty-seven percent of the horses were seropositive. Zoonoses), Animal Pathology The Arabian Pure Blood and the autochthonous Pura Raza Galega showed the highest and the lowest Department, Veterinary Faculty, exposure to horseflies, respectively. Geldings showed the maximum values of seroprevalence (96%), Santiago de Compostela University, Lugo, Spain while mares achieved the minimum. The highest percentage of seropositivity was reported in the oldest ones (72%). Significant differences were recorded (P<0.05). Our findings underline a high risk of Iván Francisco sensitization among horses to bot fly in regions with an oceanic climate. Equine Diseases Study Group (Epidemiology, Parasitology and Keywords: Gasterophilus, horse, ELISA, IgG, serological survey Zoonoses), Animal Pathology Department, Veterinary Faculty, Santiago de Compostela University, 1. -

Gasterophilus Flavipes (Oestridae: Gasterophilinae)
Københavns Universitet Gasterophilus flavipes (Oestridae: Gasterophilinae): A horse stomach bot fly brought back from oblivion with morphological and molecular evidence Li, Xin-yu; Pape, Thomas; Zhang, Dong Published in: PLoS ONE DOI: 10.1371/journal.pone.0220820 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Li, X., Pape, T., & Zhang, D. (2019). Gasterophilus flavipes (Oestridae: Gasterophilinae): A horse stomach bot fly brought back from oblivion with morphological and molecular evidence. PLoS ONE, 14(8), [0220820]. https://doi.org/10.1371/journal.pone.0220820 Download date: 06. nov.. 2019 RESEARCH ARTICLE Gasterophilus flavipes (Oestridae: Gasterophilinae): A horse stomach bot fly brought back from oblivion with morphological and molecular evidence 1,2 2 1 Xin-yu Li , Thomas PapeID , Dong ZhangID * 1 Key Laboratory of Non-Invasive Research Technology for Endangered Species, School of Nature a1111111111 Conservation, Beijing Forestry University, Beijing, China, 2 Natural History Museum of Denmark, University of Copenhagen, Copenhagen, Denmark a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 Abstract Species of Gasterophilus Leach are obligate parasites in domestic and wild equids and OPEN ACCESS responsible for cosmopolitan gasterophilosis. Although with only eight species known so far, they have received considerable attention because of their significant veterinary and Citation: Li X-y, Pape T, Zhang D (2019) Gasterophilus flavipes (Oestridae: Gasterophilinae): economic importance. Surprisingly, we found that G. flavipes (Olivier) is a valid species A horse stomach bot fly brought back from based on morphological characters from male, female and the egg, after spending half a oblivion with morphological and molecular century as a synonym of G. -

The Prevalence of Gasterophilus Intestinalis
Open Veterinary Journal, (2018), Vol. 8(4): 423-431 ISSN: 2226-4485 (Print) Original Article ISSN: 2218-6050 (Online) DOI: http://dx.doi.org/10.4314/ovj.v8i4.12 _____________________________________________________________________________________________ Submitted: 09/04/2018 Accepted: 19/10/2018 Published: 15/11/2018 The prevalence of Gasterophilus intestinalis (Diptera: Oestridae) in donkeys (Equus asinus) in Egypt with special reference to larvicidal effects of neem seed oil extract (Azadirachta indica) on third stage larvae Marwa M. Attia*, Marwa M. Khalifa and Olfat A. Mahdy Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Giza, P.O. Box 12211, Egypt _____________________________________________________________________________________________ Abstract Gasterophiline larvae are of veterinary and medical importance caused specific equine intestinal myiasis. Gasterophilus intestinalis (Botfly larvae) had a wide geographical distribution. The present study explores the prevalence rate of G. intestinalis 3rd stage larvae in Egypt from January- December 2017; besides, in vitro trials to control of this larvae and evaluation of this trial using Scanning Electron Microscope (SEM) and histopathology of treated larvae. In the present study, the 3rd larval stage of G. intestinalis was found in clusters in the epithelium of the investigated stomach and infested with prevalence rate 97.2%. The highest collected numbers of larvae were found in two months; March and August (570 & 520 larvae) and lowest numbers (200 larvae) were collected in October, November, and December. The calculated LC50 and LC90 values of neem seed extract were 707.9 ppm and 1090.7 ppm. The different alteration was recorded after exposure to oil extract which showed some destruction on cuticle surface as folded and corrugated cuticle, destruction of maxillae with pits on its surface, disfigure and irregularity of cephalic spines.