Documentation of Indigenous Antiparasitic Practices and Scientific Evaluation of Some Ethnobotanicals for Their Anthelmintic Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

347 ZTBL Branches That Shall Remain Open on Saturday W.E.F 12.09.2020 to 31.12.2020
347 ZTBL Branches that shall remain open on Saturday w.e.f 12.09.2020 to 31.12.2020 Sr. Branch Branch Name Zone Name Location/Address No. Code 1 22304 Bahawalnagar Bahawalnagar Kamboh House, Boys Degree Collge Road, Bahawalnagar 2 22353 Bahawalnagar City Bahawalnagar Grain Market, Cantt. Road, Bahawalnagar City 3 22337 Madrassa Bahawalnagar Main Chishtian Road,Madrassa 4 22329 Donga Bonga Bahawalnagar Bahawalnagar Road, Donga Bonga 5 22348 Gajyani Bahawalnagar Highway Haroonabad Road, Gajyani 6 22311 Fort Abbas Bahawalnagar Maroot Road, Near Bus Stand, Fortabbas 7 22338 Maroot Bahawalnagar High Way Road, Maroot 8 22344 Khichiwala Bahawalnagar Plot No. 57,Wahlar Road, Khichiwala. 9 22312 Haroonabad Bahawalnagar Goddi Road, Near Educare School, Haroonabad 10 22332 Fakir Wali Bahawalnagar High Way Road, Fakir Wali 11 22310 Minchinabad Bahawalnagar Pakpattan Road, Near AC Office, Minchinabad 12 22330 Ahmedpur Mclood Gunj Bahawalnagar Main Road, General Bus Stand, Ahmedpur Mclood Gunj 13 22343 Chabhyana Bahawalnagar Main Highway Road, Chabhyana 14 22349 Mandi Sadiq Gunj Bahawalnagar Amroka Road, Mandi Sadiq Gunj 15 22305 Chishtian Bahawalnagar High Way Road, (sugar Mill Road), Chishtian 16 22336 Bakhshan Khan Bahawalnagar High Way Chishtian Road, Bakhshan Khan 17 22331 Dahranwala Bahawalnagar Opposite High School for Boys, Dahranwala 18 22301 Bahawalpur Bahawalpur H No.8-A, Dubai Chowk, Ahmedpur East Road, Bahawalpur 19 22323 Noorpur Nauranga Bahawalpur Main Khanqah Road, Near Pull Shahab,Noorpur Nauranga 20 22341 Khanqah Sharif Bahawalpur -

Public Notice Auction of Gold Ornament & Valuables
PUBLIC NOTICE AUCTION OF GOLD ORNAMENT & VALUABLES Finance facilities were extended by JS Bank Limited to its customers mentioned below against the security of deposit and pledge of Gold ornaments/valuables. The customers have neglected and failed to repay the finances extended to them by JS Bank Limited along with the mark-up thereon. The current outstanding liability of such customers is mentioned below. Notice is hereby given to the under mentioned customers that if payment of the entire outstanding amount of finance along with mark-up is not made by them to JS Bank Limited within 15 days of the publication of this notice, JS Bank Limited shall auction the Gold ornaments/valuables after issuing public notice regarding the date and time of the public auction and the proceeds realized from such auction shall be applied towards the outstanding amount due and payable by the customers to JS Bank Limited. No further public notice shall be issued to call upon the customers to make payment of the outstanding amounts due and payable to JS Bank as mentioned hereunder: Customer ID Customer Name Address Amount as of 8th April 1038553 ZAHID HUSSAIN MUHALLA MASANDPURSHI KARPUR SHIKARPUR 343283.35 1012051 ZEESHAN ALI HYDERI MUHALLA SHIKA RPUR SHIKARPUR PK SHIKARPUR 409988.71 1008854 NANIK RAM VILLAGE JARWAR PSOT OFFICE JARWAR GHOTKI 65110 PAK SITAN GHOTKI 608446.89 999474 DARYA KHAN THENDA PO HABIB KOT TALUKA LAKHI DISTRICT SHIKARPU R 781000 SHIKARPUR PAKISTAN SHIKARPUR 361156.69 352105 ABDUL JABBAR FAZALEELAHI ESTATE S HOP NO C12 BLOCK 3 SAADI TOWN -
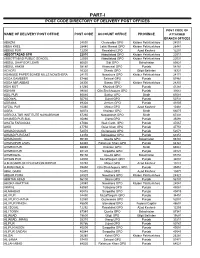
Part-I: Post Code Directory of Delivery Post Offices
PART-I POST CODE DIRECTORY OF DELIVERY POST OFFICES POST CODE OF NAME OF DELIVERY POST OFFICE POST CODE ACCOUNT OFFICE PROVINCE ATTACHED BRANCH OFFICES ABAZAI 24550 Charsadda GPO Khyber Pakhtunkhwa 24551 ABBA KHEL 28440 Lakki Marwat GPO Khyber Pakhtunkhwa 28441 ABBAS PUR 12200 Rawalakot GPO Azad Kashmir 12201 ABBOTTABAD GPO 22010 Abbottabad GPO Khyber Pakhtunkhwa 22011 ABBOTTABAD PUBLIC SCHOOL 22030 Abbottabad GPO Khyber Pakhtunkhwa 22031 ABDUL GHAFOOR LEHRI 80820 Sibi GPO Balochistan 80821 ABDUL HAKIM 58180 Khanewal GPO Punjab 58181 ACHORI 16320 Skardu GPO Gilgit Baltistan 16321 ADAMJEE PAPER BOARD MILLS NOWSHERA 24170 Nowshera GPO Khyber Pakhtunkhwa 24171 ADDA GAMBEER 57460 Sahiwal GPO Punjab 57461 ADDA MIR ABBAS 28300 Bannu GPO Khyber Pakhtunkhwa 28301 ADHI KOT 41260 Khushab GPO Punjab 41261 ADHIAN 39060 Qila Sheikhupura GPO Punjab 39061 ADIL PUR 65080 Sukkur GPO Sindh 65081 ADOWAL 50730 Gujrat GPO Punjab 50731 ADRANA 49304 Jhelum GPO Punjab 49305 AFZAL PUR 10360 Mirpur GPO Azad Kashmir 10361 AGRA 66074 Khairpur GPO Sindh 66075 AGRICULTUR INSTITUTE NAWABSHAH 67230 Nawabshah GPO Sindh 67231 AHAMED PUR SIAL 35090 Jhang GPO Punjab 35091 AHATA FAROOQIA 47066 Wah Cantt. GPO Punjab 47067 AHDI 47750 Gujar Khan GPO Punjab 47751 AHMAD NAGAR 52070 Gujranwala GPO Punjab 52071 AHMAD PUR EAST 63350 Bahawalpur GPO Punjab 63351 AHMADOON 96100 Quetta GPO Balochistan 96101 AHMADPUR LAMA 64380 Rahimyar Khan GPO Punjab 64381 AHMED PUR 66040 Khairpur GPO Sindh 66041 AHMED PUR 40120 Sargodha GPO Punjab 40121 AHMEDWAL 95150 Quetta GPO Balochistan 95151 -
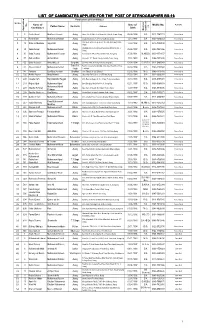
List of Candidates Applied for the Post of Stenographer Bs-16 List of Candidates Applied for the Post of Stenographer Bs-16
LIST OF CANDIDATES APPLIED FOR THE POST OF STENOGRAPHER BS-16 Particulars of Candidates Sr.No Name of Date of Mobile No. Remarks No. Domicile Address . Father Name on Candidates Birth Maximum Maximum Application Qualificati 1 5 Rabia Bassri Manzoor Hussain Jhang House No. 611/B-II, Near Main Gate, Satellite Town, Jhang 06.08.1994 B.A 0310-7547723 Women Quota 2 16 Rubina Bibi Muhammad Nawaz Jhang Mauza Mulkhiana, P.O Pir Kot Sadhana, Jhang 14.12.1995 B.A 0340-1334554 Women Quota Near Govt. Girls College House No. 234, Moh. Burji Wala, 19 Sidra-tul-Muntaha Sajjad Ali Jhang 12.02.1998 B.A 0313-7058599 Women Quota 3 Jhang Shahdab Colony Canal Road Near Sialvi Masjid St. No. 3 28 Sadia Anwar Muhammad Anwar Jhang 05.06.1997 B.A 0300-7501866 Women Quota 4 Jhang 5 30 Sidra Younas Muhammad Younas Jhang Purana Chiniot Road, Basti Shehni Wali Jhang City 25.08.1991 M.A/B.Ed 0300-3553157 Women Quota 6 57 Zaib un Nisa Ahmad Khan Jhang House No. 702, Behar Colony, Satellite Town, Jhang 15.01.1993 B.A 0346-7251636 Women Quota 7 66 Sidra Hussain Abdul Majeeb Sargodha Ijaz Photo State Nehang, Sahiwal, Sargodha 01.04.1994 B.A/B.Ed 0312-6889043 Women Quota Toba Tek Brain Cox Academy, Abdullah Chak, Akal Wala Road Toba 8 67 Adeela Ashraf Muhammad Ashraf 04.04.1994 M.A 0345-1109949 Women Quota Singh Tek Singh 9 141 Famtara Syed ijaz Hussain Bhakkar Jhok Tibba, P.O Sial, Bhakkar 02.06.1995 M.Sc 0349-7718785 Women Quota 10 192 Nabila Kauser Abdul Hamid Jhang House NO. -
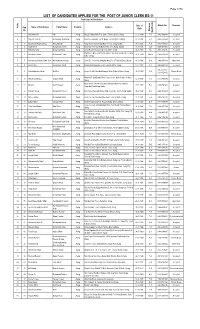
LIST of CANDIDATES APPLIED for the POST of JUNIOR CLERK BS-11 Particulars of Candidates
Page 1/156 LIST OF CANDIDATES APPLIED FOR THE POST OF JUNIOR CLERK BS-11 Particulars of Candidates Date of Mobile No. Remarks Sr.No. App. Name of Candidates Father Name Domicile Address Birth No. Maximum Qualification 1 1 Muhammad Ali Adil Jhang Mouza Pakkay Wala P.O. Same, Tehsil & District Jhang. 20.10.1994 F.A. 0342-8396398 Accepted 2 2 Wajahat Ali Khan Mohammad Sharif Khan Jhang Mauza Luck-Baddher, P.O. Bagh, Tehsil & District Jhang. 31.12.1995 F.A. 0344-0673244 Accepted 3 3 Muhammad Nauman Aeaz Aejaz Hussain Jhang Sargodha Road, Mohallah Rasool Pura, Jhang Saddar. 07.03.1996 F.A. 0346-4095001 Accepted 4 4 Asad Ameen Muhammad Ameen Jhang Basti Mai Heer near by Darbar Mai Heer, Jhang Saddar. 09.09.1996 B.A 0348-8700532 Accepted 5 5 Mubashar Iqbal Muhammad Iqbal Jhang Gojra Road, Faisal Colony House #283, Jhang 26.06.1988 Fsc 0313-7692185 Accepted Ward No.7, H/No.559/173, Mohallah Tahir Abad, Jhang City, Tehsil & 6 6 Mhammad Usman Muhammad Yousaf Jhang 05.11.1989 Master 0344-1774797 Accepted District Jhang 7 7 Muhammad Zeshan Haider Shah Haji Muhammad Hayat Jhang Street Dr. Yasin Wali, Mohallah Marzi Pura, Tehsil & District Jhang 14.08.1995 B.A. 0316-3274343 Open Merit 8 8 Aman Ullah Muhammad Ashraf Jhang Moza Habib Sargodha Road, Tehsil & District Jhang. 17.07.1992 B.A. 0343-4885147 Accepted 0345-6728052 / 9 9 Hafiza Humaira Jabeen Gul Sher Jhang House No.665, Mohallah Kapayan Wala, Tehsil & District Jhang 08.09.1994 F.A. Women Qouta 0321-6728052 Ward No.7, Gali Banwai Wali, House No.81, Mohalla Shreefa Wala 10 10 Muhammad Waqas Intazar Ahmad Jhang 12.07.1998 F.A. -

Village List of Multan Division , Pakistan
Cel'.Us 51·No. 30B (I) M.lnt.6-19 300 CENSUS OF PAKISTAN, 1951 VILLAGE LIST PUNJAB Multan Division OFFICE Of THE PROVINCIAL · .. ·l),ITENDENT CENSUS, J~ 1952 ,~ :{< 'AND BAHAWALPUR, P,IC1!iR.. 10 , , FOREWOf~D This Village Ust has been prepared from the material collected in con nection with the Census of Pakistan, 1951. The object of the List is to present useful information about our villages. It was considered that in a predominantly rural country like Pakistan, reliable village statistics should be available and it is hoped that the Village List will form the basis for the continued collection of such statistics. A summary table of the totals for each tehsil showinz its area to the nearest square mile, and its population and the number of houses to the nearest hundred is given on page I together with the page number on which each tehsil begins. The general village table, which has been compiled district-wise and arranged tehsil-wise, appears on page 3 et seq. Within each tehsll th~ Revenue Kanungo ho/qas are shown according to their order in the census records. The Village in which the Revenue Kanungo usually resides is printed in bold type at the beginning of each Kanungo halqa and the remaining villages comprising the halqas, are shown thereunder in the order of their revenue hadbast numbers, which are given in column a. Rakhs (tree plantations) and other similar area,. even where they are allotted separate revenue hadbast nurY'lbcrs have not been shown as they were not reported in the Charge and Household summaries, to be inhabited. -
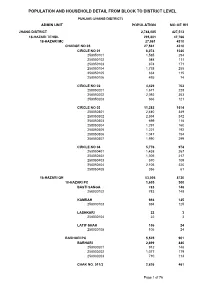
Jhang Blockwise
POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (JHANG DISTRICT) ADMIN UNIT POPULATION NO OF HH JHANG DISTRICT 2,744,085 427,513 18-HAZARI TEHSIL 295,801 47,766 18-HAZARI MC 27,561 4310 CHARGE NO 05 27,561 4310 CIRCLE NO 01 6,074 1020 258050101 1,585 294 258050102 548 111 258050103 874 171 258050104 1,738 255 258050105 834 115 258050106 495 74 CIRCLE NO 02 4,429 702 258050201 1,671 228 258050202 2,092 353 258050203 666 121 CIRCLE NO 03 11,282 1614 258050301 2,440 349 258050302 2,594 342 258050303 699 118 258050304 1,291 160 258050305 1,221 192 258050306 1,047 154 258050307 1,990 299 CIRCLE NO 04 5,776 974 258050401 1,438 267 258050402 1,306 217 258050403 570 109 258050404 2,106 320 258050405 356 61 18-HAZARI QH 53,006 8720 18-HAZARI PC 1,605 300 BASTI SANGA 783 148 258030102 783 148 KAMRAH 694 125 258030103 694 125 LASHKARI 22 3 258030104 22 3 LATIF SHAH 106 24 258030105 106 24 BARHARI PC 5,525 901 BARHARI 2,699 440 258030201 912 148 258030202 1,077 179 258030203 710 113 CHAK NO. 011/3 2,826 461 Page 1 of 76 POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (JHANG DISTRICT) ADMIN UNIT POPULATION NO OF HH 258030204 1,317 197 258030205 1,509 264 DAL PC 4,738 708 DAL 4,738 708 258030301 1,171 187 258030302 1,634 239 258030303 1,933 282 DHABBI PC 1,923 326 DHABBI 1,174 188 258030401 1,174 188 LUHRKA 449 89 258030404 449 89 SARWANI PATOANA 300 49 258030405 300 49 KOT NAULAN PC 5,142 937 FATEH SHAH 920 157 258030504 920 157 KOT NAULAN 4,096 755 258030501 2,510 465 258030502 1,586 290 SHAH MAHMUD 126 25 -

Punjab ! ! Overview ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! - PUNJAB ! ! OVERVIEW ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! KHYBER ! ! ! ! ! PAKHTUNKHWA ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Chamba Pind ! ! ! Attock ! ! ! Hazro ! ! ! Murree ! ! ! ! Bhabra PAK ! ! ! ! ! AttockBura Hassan Abdal ! ! Wah ! Amanpura ! ! ! ! !!! ! Kotli Sattian ! A!ttock ! Bhangal ! Taxila ! ! FATA Akhori Bahlol ! ! ! ! Autrinna Mariala Bhatiot Badhana Kalan ! ! ! ! ! Rawalpindi ! ! ! ! ! !! Rawalpindi Kahuta ! Fatehjang ! ! ! Basal ! ! Morgah JAMMU & KASHMIR ! Jalwal Bango ! ! Achhral ! Band ! ! Murat ! ! Rangli Fateh Jang ! ! ATTOCK ! Adiala Gali Jagir ! Jand Abawal Bagh ! ! Bhunan Wali ! Dulehal ! ! Kallar Sayedan ! ! ! ! Bagra Arazi Chhur Mall Choha Khalsa ! ! Rawalpindi ! Jand Mandra ! !Ghalwal ! Dhok Ganganwali ! ! Malikpur ! Pari Kali ! Jhamat Dabhula ! ! Malangi ! ! Ahmadal Balawal ! ! ! ! ! ! RAWALPINDI Gujar Khan ! Saura ! Ratala ! ! Pindi Gheb ! ! Chak Beli ! !Pindi Gheb !Maghian ! Gujar Khan ! ! Malal ! ! Maira Behkhari ! ! Neela ! ! Hadawali ! ! ! Bahwaley Kallan Dora Badhal ! Dhok Afghan ! Adhi ! Makhad ! Visor ! ! ! Banth Pandori ! ! ! Dhok Abakki ! ! ! ! Shah Muhammad Wali Dewal Jamalwal Dhudial Arazi Hamid ! Bor Khui ! ! ! ! Hasola ! ! ! Multan ! ! -

Jhang National Assembly Polling Scheme 2018
ELECTION COMMISSION OF PAKISTAN FORM-28 [see rule 50] DRAFT LIST OF POLLING STATIONS FOR A CONSTITUENCY OF Election to the National Assembly of the NA-114 JHANG-I S. No. of voters Number of voters assigned Number of polling In Case of Rural Areas In Case of Urban Areas on the to polling station booths No. and Name of Polling electoral roll in Sr. No. Station case electoral Census Block Census Block Name of Electoral Areas Name of Electoral Areas area is Male Female Total Male Female Total Code Code bifurcated 1 2 3 4 5 6 7 8 9 10 11 12 13 Government Boys Primary Bali 156070101 - - 504 345 849 1 2 1 3 School Balian Bali 156070102 - - 239 134 373 1 Total - - - 743 479 1222 2 1 3 Government Boys Primary 2 Punjgarain 156070201 - - 695 540 1235 1 1 2 School Punjgarain 2 Total - - - 695 540 1235 1 1 2 Punjgarain 156070202 - - 420 273 693 3 Basic Health Unit Panjgarain 1 1 2 Punjgarain 156070203 - - 195 154 349 3 Total - - - 615 427 1042 1 1 2 Government Girls Community Model School, 4 Tahatta Jhabana 156070301 - - 969 717 1686 2 2 4 Hussain Abad Thatha Jhabana 4 Total - - - 969 717 1686 2 2 4 Government Boys Elementry 5 Tahatta Jhabana 156070302 - - 708 517 1225 2 1 3 School Tahatta Jhabana 5 Total - - - 708 517 1225 2 1 3 Government Girls Primary 6 Murad Wala 156070303 - - 432 291 723 1 1 2 School Chak Murad Murad 6 Total - - - 432 291 723 1 1 2 Government Boys Primary Thatta Korriana 156070401 - - 348 291 639 7 1 1 2 School Thatta Sundrana Thatta Korriana 156070403 - - 231 88 319 7 Total - - - 579 379 958 1 1 2 1 S. -
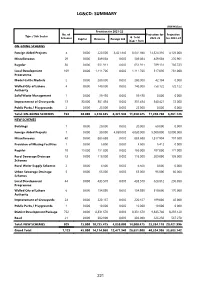
Lg&Cd: Summary
LG&CD: SUMMARY (PKR Million) Provision for 2021-22 No. of Projection for Projection Type / Sub Sector G. Total Schemes Capital Revenue Foreign Aid 2022-23 for 2023-24 (Cap + Rev) ON-GOING SCHEMES Foreign Aided Projects 4 0.000 220.000 8,421.940 8,641.940 14,324.310 4,129.000 Miscellaneous 29 0.000 589.084 0.000 589.084 469.604 270.901 Regular 30 0.000 651.911 0.000 651.911 789.131 786.723 Local Development 109 0.000 1,111.706 0.000 1,111.706 517.096 281.000 Programme Model Cattle Markets 2 0.000 280.000 0.000 280.000 42.104 0.000 Walled City of Lahore 4 0.000 140.000 0.000 140.000 758.122 722.122 Authority Solid Waste Management 1 0.000 59.430 0.000 59.430 0.000 0.000 Improvement of Graveyards 13 30.000 301.454 0.000 331.454 340.421 72.000 Public Parks / Playgrounds 2 0.000 25.000 0.000 25.000 0.000 0.000 Total: ON-GOING SCHEMES 194 30.000 3,378.585 8,421.940 11,830.525 17,240.788 6,261.746 NEW SCHEMES Buildings 1 0.000 20.000 0.000 20.000 60.000 0.000 Foreign Aided Projects 1 0.000 30.000 4,050.000 4,080.000 9,000.000 19,000.000 Miscellaneous 40 0.000 885.688 0.000 885.688 1,817.904 797.500 Provision of Missing Facilities 1 0.000 5.000 0.000 5.000 5.412 0.000 Regular 18 15.000 151.000 0.000 166.000 287.000 121.000 Rural Sewerage Drainage 13 0.000 118.000 0.000 118.000 209.000 105.000 Schemes Rural Water Supply Schemes 2 0.000 6.500 0.000 6.500 0.000 0.000 Urban Sewerage Drainage 5 0.000 65.000 0.000 65.000 95.000 60.000 Schemes Local Development 44 0.000 435.570 0.000 435.570 628.812 296.098 Programme Walled City of Lahore 6 0.000 154.880 0.000 154.880 510.000 170.000 Authority Improvement of Graveyards 24 0.000 220.167 0.000 220.167 189.000 46.000 Public Parks / Playgrounds 1 0.000 10.000 0.000 10.000 10.000 0.000 District Development Package 752 0.000 8,331.670 0.000 8,331.670 9,845.740 8,259.128 Road 21 0.000 302.000 0.000 302.000 626.250 567.270 Total: NEW SCHEMES 929 15.000 10,735.475 4,050.000 14,800.475 23,284.118 29,421.996 Grand Total 1,123 45.000 14,114.060 12,471.940 26,631.000 40,524.906 35,683.742 221 LG&CD (PKR Million) Accum. -

List of Agro Based Industry in the District Lahore
LIST OF AGRO BASED INDUSTRY IN THE DISTRICT LAHORE Sr. Agro Based Complete Address Fax No./ E-mail Phone No./Mobile Name of Factory/Mill Website No. Industry (Tehsil/District) Address No. supremfloor@supreem 1 Supreme flour Mill 22 Km Multan Road Lahore - 04235979103-4 .com.pk 042-37417541, 2 Ferdous flour Mill Band Road Bakar Mandi Lahore - - 042-37417535 Ghulamhussain0009@ 0323-9000349, 3 Good Luck flour Mill Defiance Road Mohnal wal Lahore - gmail.com 0323-900689 4 Sajid flour Mill Defiance Road Mohnal wal Lahore - - 0333-4785186 yaqomiafloorMill@gmai 0300-4104232, 5 Yaqomia flour Mill Pool LubanaMultan Road Lahore - l.com 04237511216-17-18 6 zimidaar flour Mill Sundar Indusial Sundar Raiwind - - 0300-8445300 Sundar Indusial Sundar Raiwind 0321-4855526, 7 Arabia flour Mill - - Road Lahore 042-35063394 8 Farman flour Mill Sham Ky Bhattia Multan road Lahore - - 0321-4203006 18Km Multan Road Near Shabab 9 Hajvery flour Mill - - 3238117777 Stoudo Block No.394 Sundar Indusial State abullahfloorMill@yahoo 0300-8499467, 10 Flour Mills Abdullah flour Mill - Sundar Raiwind Road .com 04235298519 Sundar Indusial State Sundar 11 Bahoo flour Mill - - 0308-6666888 Raiwind Road Block No.37 Sundar Indusial State 0321-7777466, 12 Al-Muhktar flour Mill www.mukhtar.pk - Sundar Raiwind Road 0300-8465151 Sundar Indusial State Sundar 13 Taaj flour Mill - - 0301-6577037 Raiwind Road Lahore Mouza sultan kay Sundar Raiwind 14 Ajwa flour Mill - - 0300-6561208 Road lahore Malik M.Rafoq S/o Abdul Aziz 15 Malik flour Mill - - 0320-3414121 Sundar Raiwind Road Lahore 16 Number Daar flour Mill - - 0333-4508002 17 Raiwind Flour Mills Sunder Road Raiwind Dist Lahore - - 042-5390998 18 Behria Flour Mills Lahore Road Raiwind Dist Lahore - - 0300-8482012 19 G.L Flour Mills Lahore Road Raiwind Dist Lahore - - 0303-4563077 Sr. -
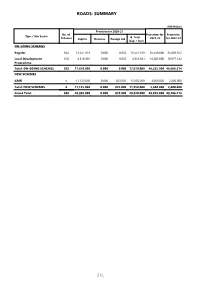
Roads: Summary
ROADS: SUMMARY (PKR Million) Provision for 2020-21 No. of Projection for Projection Type / Sub Sector G. Total Schemes Capital Revenue Foreign Aid 2021-22 for 2022-23 (Cap + Rev) ON-GOING SCHEMES Regular 364 13,551.619 0.000 0.000 13,551.619 35,550.000 35,889.072 Local Development 518 4,318.381 0.000 0.000 4,318.381 10,685.000 10,977.142 Programme Total: ON-GOING SCHEMES 882 17,870.000 0.000 0.000 17,870.000 46,235.000 46,866.214 NEW SCHEMES C&W 6 11,125.000 0.000 825.000 11,950.000 3,600.000 2,400.000 Total: NEW SCHEMES 6 11,125.000 0.000 825.000 11,950.000 3,600.000 2,400.000 Grand Total 888 28,995.000 0.000 825.000 29,820.000 49,835.000 49,266.214 261 Roads (PKR Million) Accum. Provision for 2020-21 MTDF Projections Throw fwd GS Scheme Information Est. Cost Exp. G.Total Beyond No Scheme ID / Approval Date / Location Cap. Rev. 2021-22 2022-23 June, 20 (Cap.+Rev.) June, 2023 1 2 3 4 5 6 7 8 9 10 ON-GOING SCHEMES Regular 2070 Rehabilitation of road from Bansra Gali 528.091 438.940 5.000 0.000 5.000 42.000 42.151 0.000 to Lawrance collage to Jhika Gali, L- 9.35 kms 01291300207 / 01-07-2013 / Rawalpindi 2071 Widening / Improvement of road from 96.764 61.826 3.000 0.000 3.000 16.000 15.938 0.000 Kotli Sattian milad Chowk to Bhan, L= 8.43 KM Tehsil Kotli Sattian 01291501812 / 01-07-2015 / Rawalpindi 2072 Construction of road Kahuti to Kulyari, 364.111 66.062 70.000 0.000 70.000 134.000 134.049 0.000 Length 31.00 Km.