E.D. Houde and E.S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flood Insurance Study
FLOOD INSURANCE STUDY CECIL COUNTY, MARYLAND AND INCORPORATED AREAS Cecil County Community Community Name Number ↓ CECIL COUNTY (UNINCORPORATED AREAS) 240019 *CECILTON, TOWN OF 240020 CHARLESTOWN, TOWN OF 240021 CHESAPEAKE CITY, TOWN OF 240099 ELKTON, TOWN OF 240022 NORTH EAST, TOWN OF 240023 PERRYVILLE, TOWN OF 240024 PORT DEPOSIT, TOWN OF 240025 RISING SUN, TOWN OF 240158 *No Special Flood Hazard Areas Identified Revised: May 4, 2015 Federal Emergency Management Agency FLOOD INSURANCE STUDY NUMBER 24015CV000B NOTICE TO FLOOD INSURANCE STUDY USERS Communities participating in the National Flood Insurance Program (NFIP) have established repositories of flood hazard data for floodplain management and flood insurance purposes. This Flood Insurance Study (FIS) report may not contain all data available within the Community Map Repository. Please contact the Community Map Repository for any additional data. Part or all of this FIS may be revised and republished at any time. In addition, part of the FIS may be revised by the Letter of Map Revision (LOMR) process, which does not involve republication or redistribution of the FIS. It is, therefore, the responsibility of the user to consult with community officials and to check the community repository to obtain the most current FIS components. Initial Countywide FIS Effective Date: July 8, 2013 Revised Countywide FIS Effective Date: May 4, 2015 TABLE OF CONTENTS Page 1.0 INTRODUCTION ............................................................................................................. -

Maryland Stream Waders 10 Year Report
MARYLAND STREAM WADERS TEN YEAR (2000-2009) REPORT October 2012 Maryland Stream Waders Ten Year (2000-2009) Report Prepared for: Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 1-877-620-8DNR (x8623) [email protected] Prepared by: Daniel Boward1 Sara Weglein1 Erik W. Leppo2 1 Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 2 Tetra Tech, Inc. Center for Ecological Studies 400 Red Brook Boulevard, Suite 200 Owings Mills, Maryland 21117 October 2012 This page intentionally blank. Foreword This document reports on the firstt en years (2000-2009) of sampling and results for the Maryland Stream Waders (MSW) statewide volunteer stream monitoring program managed by the Maryland Department of Natural Resources’ (DNR) Monitoring and Non-tidal Assessment Division (MANTA). Stream Waders data are intended to supplementt hose collected for the Maryland Biological Stream Survey (MBSS) by DNR and University of Maryland biologists. This report provides an overview oft he Program and summarizes results from the firstt en years of sampling. Acknowledgments We wish to acknowledge, first and foremost, the dedicated volunteers who collected data for this report (Appendix A): Thanks also to the following individuals for helping to make the Program a success. • The DNR Benthic Macroinvertebrate Lab staffof Neal Dziepak, Ellen Friedman, and Kerry Tebbs, for their countless hours in -

Christina River Wetland Condition Report
Matthew A. Jennette Alison B. Rogerson Andrew M. Howard Delaware Department of Natural Resources and Environmental Control Division of Watershed Stewardship 820 Silver Lake Blvd, Suite 220 Dover, DE 19904 LeeAnn Haaf Danielle Kreeger Angela Padeletti Kurt Cheng Jessie Buckner Partnership for the Delaware Estuary 110 South Poplar Street, Suite 202 Wilmington, DE 19801 The citation for this document is: Jennette, M.A., L. Haaf, A.B. Rogerson, A.M. Howard, D. Kreeger, A. Padeletti, K. Cheng, and J. Buckner. 2014. Condition of Wetlands in the Christiana River Watershed. Delaware Department of Natural Resources and Environmental Control, Watershed Assessment and Management Section, Dover, DE and the Partnership for the Delaware Estuary, Wilmington, DE. ii Christina River Wetland Condition ACKNOWLEDGEMENTS Funding for this project was provided by EPA Region III Wetland Program Development Grant Assistance # CD-96312201-0, EPA National Estuary Program, the Delaware Department of Natural Resources, and the DuPont Clear into the Future program. Technical support and field research was made possible by the contributions of many individuals. Tracy Quirk and Melanie Mills from the Academy of Natural Science of Drexel University (ANS) for the collection and analysis of Site Specific data, and assistance with RAM data collection and analysis. Tom Kincaid and Tony Olsen with the EPA Office of Research and Development Lab, Corvallis, Oregon provided the data frame for field sampling and statistical weights for interpretation. Kristen Kyler and Rebecca Rothweiler participated in many field assessments under a myriad of conditions. Maggie Pletta and Michelle Lepori-Bui were helpful with revisions on drafts of the report, as well as producing the cover art for the final document. -

Fishing Regulations JANUARY - DECEMBER 2004
WEST VIRGINIA Fishing Regulations JANUARY - DECEMBER 2004 West Virginia Division of Natural Resources D I Investment in a Legacy --------------------------- S West Virginia’s anglers enjoy a rich sportfishing legacy and conservation ethic that is maintained T through their commitment to our state’s fishery resources. Recognizing this commitment, the R Division of Natural Resources endeavors to provide a variety of quality fishing opportunities to meet I increasing demands, while also conserving and protecting the state’s valuable aquatic resources. One way that DNR fulfills this part of its mission is through its fish hatchery programs. Many anglers are C aware of the successful trout stocking program and the seven coldwater hatcheries that support this T important fishery in West Virginia. The warmwater hatchery program, although a little less well known, is still very significant to West Virginia anglers. O West Virginia’s warmwater hatchery program has been instrumental in providing fishing opportunities F to anglers for more than 60 years. For most of that time, the Palestine State Fish Hatchery was the state’s primary facility dedicated to the production of warmwater fish. Millions of walleye, muskellunge, channel catfish, hybrid striped bass, saugeye, tiger musky, and largemouth F and smallmouth bass have been raised over the years at Palestine and stocked into streams, rivers, and lakes across the state. I A recent addition to the DNR’s warmwater hatchery program is the Apple Grove State Fish Hatchery in Mason County. Construction of the C hatchery was completed in 2003. It was a joint project of the U.S. Army Corps of Engineers and the DNR as part of a mitigation agreement E for the modernization of the Robert C. -

A Summary of Peak Stages Discharges in Maryland, Delaware
I : ' '; Ji" ' UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY · Water Resources Division A SUMMARY OF PEAK STAGES AND DISCHARGES IN MARYLAND, DELAWARE, AND DISTRICT OF COLUMBIA FOR FLOOD OF JUNE 1972 by Kenneth R. Taylor Open-File Report Parkville, Maryland 1972 A SUJ1D11ary of Peak Stages and Discharges in Maryland, Delaware, and District of Columbia for Flood of June 1972 by Kenneth R. Taylor • Intense rainfall associated with tropical storm Agnes caused devastating flooding in several states along the Atlantic Seaboard during late June 1972. Storm-related deaths were widespread from Florida to New York, and public and private property damage has been estllna.ted in the billions of dollars. The purpose of this report is to make available to the public as quickly as possible peak-stage and peak-discharge data for streams and rivers in Maryland, Delaware, and the District of Columbia. Detailed analyses of precipitation, flood damages, stage hydrographs, discharge hydrographs, and frequency relations will be presented in a subsequent report. Although the center of the storm passed just offshore from Maryland and Delaware, the most intense rainfall occurred in the Washington, D. c., area and in a band across the central part of Maryland (figure 1). Precipitation totals exceeding 10 inches during the June 21-23 period • were recorded in Baltimore, Carroll, Cecil, Frederick, Harford, Howard, Kent, Montgomery, and Prince Georges Counties, Md., and in the District of Columbia. Storm totals of more than 14 inches were recorded in Baltimore and Carroll Counties. At most stations more than 75 percent of the storm rainfall'occurred on the afternoon and night of June 21 and the morning of June 22 (figure 2). -
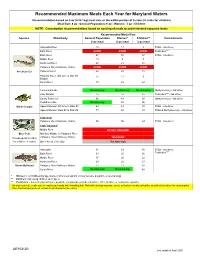
Recommended Maximum Fish Meals Each Year For
Recommended Maximum Meals Each Year for Maryland Waters Recommendation based on 8 oz (0.227 kg) meal size, or the edible portion of 9 crabs (4 crabs for children) Meal Size: 8 oz - General Population; 6 oz - Women; 3 oz - Children NOTE: Consumption recommendations based on spacing of meals to avoid elevated exposure levels Recommended Meals/Year Species Waterbody General PopulationWomen* Children** Contaminants 8 oz meal 6 oz meal 3 oz meal Anacostia River 15 11 8 PCBs - risk driver Back River AVOID AVOID AVOID Pesticides*** Bush River 47 35 27 PCBs - risk driver Middle River 13 9 7 Northeast River 27 21 16 Patapsco River/Baltimore Harbor AVOID AVOID AVOID American Eel Patuxent River 26 20 15 Potomac River (DC Line to MD 301 1511 9 Bridge) South River 37 28 22 Centennial Lake No Advisory No Advisory No Advisory Methylmercury - risk driver Lake Roland 12 12 12 Pesticides*** - risk driver Liberty Reservoir 96 48 48 Methylmercury - risk driver Tuckahoe Lake No Advisory 93 56 Black Crappie Upper Potomac: DC Line to Dam #3 64 49 38 PCBs - risk driver Upper Potomac: Dam #4 to Dam #5 77 58 45 PCBs & Methylmercury - risk driver Crab meat Patapsco River/Baltimore Harbor 96 96 24 PCBs - risk driver Crab "mustard" Middle River DO NOT CONSUME Blue Crab Mid Bay: Middle to Patapsco River (1 meal equals 9 crabs) Patapsco River/Baltimore Harbor "MUSTARD" (for children: 4 crabs ) Other Areas of the Bay Eat Sparingly Anacostia 51 39 30 PCBs - risk driver Back River 33 25 20 Pesticides*** Middle River 37 28 22 Northeast River 29 22 17 Brown Bullhead Patapsco River/Baltimore Harbor 17 13 10 South River No Advisory No Advisory 88 * Women = of childbearing age (women who are pregnant or may become pregnant, or are nursing) ** Children = all young children up to age 6 *** Pesticides = banned organochlorine pesticide compounds (include chlordane, DDT, dieldrin, or heptachlor epoxide) As a general rule, make sure to wash your hands after handling fish. -

Center for Excellence in Disabilities at West Virginia University, Robert C
ACKNOWLEDGEMENTS This publication was made possible by the support of the following organizations and individuals: Center for Excellence in Disabilities at West Virginia University, Robert C. Byrd Health Sciences Center West Virginia Assistive Technology System (WVATS) West Virginia Division of Natural Resources West Virginia Division of Tourism Partnerships in Assistive Technologies, Inc. (PATHS) Special thanks to Stephen K. Hardesty and Brittany Valdez for their enthusiasm while working on this Guide. 1 Table of Contents INTRODUCTION .......................................................... 3 • How to Use This Guide ......................................... 4 • ADA Sites .............................................................. 5 • Types of Fish ......................................................... 7 • Traveling in West Virginia ...................................... 15 COUNTY INDEX .......................................................... 19 ACTIVITY LISTS • Public Access Sites ............................................... 43 • Lakes ..................................................................... 53 • Trout Fishing ......................................................... 61 • River Float Trips .................................................... 69 SITE INDEX ................................................................. 75 SITE DESCRIPTIONS .................................................. 83 APPENDICES A. Recreation Organizations ......................................207 B. Trout Stocking Schedule .......................................209 -

Watersheds.Pdf
Watershed Code Watershed Name 02130705 Aberdeen Proving Ground 02140205 Anacostia River 02140502 Antietam Creek 02130102 Assawoman Bay 02130703 Atkisson Reservoir 02130101 Atlantic Ocean 02130604 Back Creek 02130901 Back River 02130903 Baltimore Harbor 02130207 Big Annemessex River 02130606 Big Elk Creek 02130803 Bird River 02130902 Bodkin Creek 02130602 Bohemia River 02140104 Breton Bay 02131108 Brighton Dam 02120205 Broad Creek 02130701 Bush River 02130704 Bynum Run 02140207 Cabin John Creek 05020204 Casselman River 02140305 Catoctin Creek 02130106 Chincoteague Bay 02130607 Christina River 02050301 Conewago Creek 02140504 Conococheague Creek 02120204 Conowingo Dam Susq R 02130507 Corsica River 05020203 Deep Creek Lake 02120202 Deer Creek 02130204 Dividing Creek 02140304 Double Pipe Creek 02130501 Eastern Bay 02141002 Evitts Creek 02140511 Fifteen Mile Creek 02130307 Fishing Bay 02130609 Furnace Bay 02141004 Georges Creek 02140107 Gilbert Swamp 02130801 Gunpowder River 02130905 Gwynns Falls 02130401 Honga River 02130103 Isle of Wight Bay 02130904 Jones Falls 02130511 Kent Island Bay 02130504 Kent Narrows 02120201 L Susquehanna River 02130506 Langford Creek 02130907 Liberty Reservoir 02140506 Licking Creek 02130402 Little Choptank 02140505 Little Conococheague 02130605 Little Elk Creek 02130804 Little Gunpowder Falls 02131105 Little Patuxent River 02140509 Little Tonoloway Creek 05020202 Little Youghiogheny R 02130805 Loch Raven Reservoir 02139998 Lower Chesapeake Bay 02130505 Lower Chester River 02130403 Lower Choptank 02130601 Lower -

Regulations Summary 2019
WEST VIRGINIA FISHINGRegulations Summary 2019 wvdnr.gov From the Director Last year the DNR released an updated, online interactive map that provides valuable information on all aspects of fishing and hunting adventures. DNR personnel are continuing to update information and produce new, useful maps. After hearing about the need from anglers in an online survey, DNR personnel collected lake depth data and processed new bathymetry maps for 35 lakes across the state. These maps are now available on the interactive fishing map and downloadable to print or take with you on your mobile device. Also, anglers can now access the real-time streamflow conditions from the U.S. Geological Survey on our interactive fishing map. The real-time information allows anglers to check on flow and make decisions about whether fishing conditions are ideal before heading out to a stream or river. Visit wvdnr.gov/gis for more details and links to the interactive map and other map pages. You helped fund this project through the Sport Fish Restoration Program, using excise taxes on selected fishing equipment and boat fuel. I encourage you to take advantage of West Virginia’s abundant natural resources and go fishing every chance you get. And take a friend or family member with you. It’s a great way to relax and enjoy each other’s company. Stephen S. McDaniel, DNR Director DISTRICT OFFICES Main Office - South Charleston, WV 25303 324 4th Avenue Fish Management − Mark T. Scott (304) 558-2771 Law Enforcement − Col. Jerry Jenkins (304) 558-2784 License Unit − Michael Ingram (304) 558-2758 District 1 - Farmington, WV 26571 1110 Railroad Street (304) 825-6787 Fish Management − Dave Wellman Law Enforcement − Capt. -

REVISED USDA Rural Development Is Conduct
NOTICE OF CHANGES TO ELIGIBLE AREA MAPS FOR USDA RURAL DEVELOPMENT HOUSING PROGRAMS - REVISED USDA Rural Development is conducting a review of all areas under its jurisdiction to identify areas that no longer qualify as rural for housing programs. The last rural area reviews were performed in 2012-2013 using the 2010 census data. This review, which is done every five years, will utilize the 2015 American Community Survey (ACS) data. The public shall have 90 days from December 1, 2017 (the date of this public notice) to submit comments regarding the potential loss of eligibility for Rural Development housing programs. Comments should be sent to Brad King ([email protected]). For details, or questions about specific changes, please contact the Delaware/Maryland Rural Development Housing Program staff at (301) 797-0500 ext. 4. Based on the 2015 ACS data and rural area guidance located in Handbook HB-1-3550, Chapter 5, the rural eligibility designation is under review for the following areas: Delaware 1) New Castle County – Middletown Area a. Ineligible area to begin at Rt. 301 (Summit Ridge Road/SR-71) and Armstrong Corner Road. Heading west on Armstrong Corner Road to Rt. 15 (Choptank Road). Go south on Rt. 15 (Choptank Road) to Bunker Hill Road. Go west on Bunker Hill Road to the DE/MD state line. Follow the DE/MD state line south to Strawberry Lane. Go east on Strawberry Lane to Levels Road. Follow Levels Road south to Gears Corner Road to the Appoquinimink River (Wiggins Mill Pond). Follow the Appoquinimink River (Wiggins Mill Pond) east to where it intersects with Wiggins Mill Road. -

Bay Grasses Backgrounder
Bay Grasses Backgrounder - Page 1 of 5 Upper Bay Zone In the Upper Bay Zone (21 segments extending south from the Susquehanna River to the Bay Bridge), underwater bay grasses increased 21 percent from 18,922 acres in 2007 to 22,954 acres in 2008. Ten of the 21 segments increased by at least 20 percent and by at least 12 acres from 2007 totals: 21%, Northern Chesapeake Bay Segment: 1,833 acres (2007) vs. 1,008 acres (2008) 21%, Northern Chesapeake Bay Segment 2: 11,725 acres (2007) vs. 14,194 acres (2008) 59%, Northeast River: 116 acres (2007) vs. 183 acres (2008) 70%, Upper Chesapeake Bay: 373 acres (2007) vs. 633 acres (2008) 50%, Sassafras River Segment: 1,368 acres (2007) vs. 551 acres (2008) 1204%, Sassafras River Segment 2: 5 acres (2007) vs. 52 acres (2008) 40%, Gunpowder River Segment 1: 558 acres (2007) vs. 783 acres (2008) 50%, Middle River: 551 acres (2007) vs. 828 acres (2008) 53%, Upper Central Chesapeake Bay: 124 acres (2007) vs. 188 acres (2008) 23%, Lower Chester River: 67 acres (2007) vs. 84 acres (2008) None of the 21 segments decreased by at least 20 percent and by at least 12 acres from 2007 totals. Two of the 21 segments remained unvegetated in 2008. Acres of SAV & Goal Attained Upper Chesapeake Bay Region Segment 2007 2008 Restoration Goal 2008 % of goal Northern Chesapeake Bay Segment 1 834 1007 754 134 Northern Chesapeake Bay Segment 2 11726 14194 12149 117 Northern Chesapeake Bay Total 12560 15202 12903 118 Northeast River 115 183 89 205 Elk River Segment 1 1590 1907 1844 103 Elk River Segment 2 391 440 190 232 -

Potomac Basin Comprehensive Water Resources Plan
One Basin, One Future Potomac River Basin Comprehensive Water Resources Plan Prepared by the Interstate Commission on the Potomac River Basin - 2018 - Dedication i VISION This plan provides a roadmap to achieving our shared vision that the Potomac River basin will serve as a national model for water resources management that fulfills human and ecological needs for current and future generations. The plan will focus on sustainable water resources management that provides the water quantity and quality needed for the protection and enhancement of public health, the environment, all sectors of the economy, and quality of life in the basin. The plan will be based on the best available science and data. The ICPRB will serve as the catalyst for the plan's implementation through an adaptive process in collaboration with partner agencies, institutions, organizations, and the public. Dedication ii DEDICATION This plan is dedicated to Herbert M. Sachs, an ICPRB Maryland Commissioner and Executive Director. He also served for decades with the Maryland Department of Natural Resources and the Department of the Environment. He passed away in 2017. During his nearly 30-year tenure of service to ICPRB, Sachs led the agency’s efforts in supporting the regional Chesapeake Bay Program; reallocation of water supplies, cooperative law enforcement, and water quality efforts at Jennings Randolph Reservoir; and establishment of the North Brach Potomac Task Force, a group of stakeholders and officials facilitated by ICPRB that gives stakeholders a voice in management decisions. He also was at the helm when ICPRB embarked on its successful effort to restore American shad to the Potomac River.