Alaska Arctic Marine Fish Ecology Catalog
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Noatak National Arctic Range, Alaska and Associated Area of Ecological Concern, Prepared for Fish An
~t·. tv\ ,.! " .., / ANADROMOUS FISH INVENTORY NOATAK NATIONAL ARCTIC RANGE, ALASKA and Associated Area of Ecological Concern I. Pre pared for Fish and Wildlife Service by Acetic Environmenta 1 Information and Data Center University of Alaska, Anchorage September 1975 Alaska Resotlrces 99503 Library InfonY'.ett\011 .Services " ... '-c; ~.---_:·1~ ... ~:-;~,·~·:.: - -~ • Anadromous Fish Inventory . Information Framework a. Bibliography The files of the Arctic Environmental Information and Data Center were utilized for the compilation of an initial bibliography. Refcrenc:ed titles were then obtained and citations pertaining to the area and species of interest which appeared in these reports were added to expand the initial bibliography. References were deleted if, when obtained, the study was not found to pertain to the area or species of interest. lQ a few cases where references were unobtainable, such citations are followed by the note "(not seen)" to indicate that any pertinent data contained in this reference is not included in the remainder of the inventory. All possible reference sources are listed with the exception of those containing extremely general subject matter, most early (before 1910) explor- atory reports, and annual report series such as Alaska Fishery and Jur-Seal Industries in (yeat) which were issued prior to 1960. b. Species Lists A list of anadromous and coastal marine fishes for each proposed refuge or proposed additions to existing refuges was compiled. An initial list was taken from each final environmental statement; however, three major taxonomic references were consulted to add to, or delete from this initial list - List of Fishes of Alaska and Adjacent Waters with a Guide to Some of Their Liter~ture (Quast and Hall 1972), Pacific/ Fishes of Canada (Hart 1973), and Freshwnt~r Flshes of Cannda (Scott and Crossman 1973). -
Schedule Onlinepdf
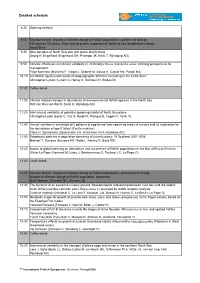
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

Arctic Science Summit Week Assw 2009
ARCTIC SCIENCE SUMMIT WEEK ASSW 2009 Bergen, Norway, 22-28 March 2009 Welcome to ASSW 2009 In 1999, the first Arctic Science Summit Week (ASSW) was organized in Tromsø, Norway. This summit was set up to hold the meetings of all Arctic science organizations together on one location in one week. The platform was created to provide opportunities for international coordination, collaboration and cooperation in all disciplines of Arctic science. It was meant to combine science with management meetings. In this way business meetings were combined with a science and a project day, and the host country was offered the opportunity to present its most recent Arctic research. Side meetings organized by other groups with interest in Arctic science and policy took place at the same time. Now, after ten ASSWs organized in ten different countries all over the world, the organizations behind this event decided to change the concept of the week by including not only annual meetings of the Arctic science organizations, but also an Open Science Symposium. Instead of the science and project day, a three days international symposium was organized under the ambitious theme: Arctic Connections - results of 150 years of Arctic research. It is the intention of the organizers to make this the official symposium for International Arctic Science. We hope that the model we have chosen for the symposium will give you the possibility to get an impression of the wide specter of research activities that have taken place in the Arctic throughout the history. After ten years the ASSW is back in Norway. -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Temperature Preference of Juvenile Arctic Cisco
TEMPERATURE PREFERENCE OF JUVENILE ARCTIC CISCO (COREGONUS AUTUMNALIS) FROM THE ALASKAN BEAUFORT SEA, IN RELATION TO SALINITY AND TEM PERATURE ACCLI~~TION by Robert G. Fechhelm LGL Ecological Research Associates, Inc. 1410 Cavitt Street Bryan, Texas 77081 William H. Neill Department of Wildlife and Fisheries Sciences Texas A&M University College Station, Texas 77843 and Benny J . Gallaway LGL Ecological Research Associates . Inc. 1410 Cavitt Street Bryan, Texas 77801 . 1 h lA flG I' ~ ;"riC 11"-' AN,) _H 707 ;.. SHiH AJ"C}1C<AGE. AI( 9'501 March 1982 2 ACKNOWLEDGEM ENTS We wish to express our gratitude to Dave Norton of the Outer Cont inental Shelf Environmental Assessment Program's Arctic Projects Office (or his support during all phases of the research. Thanks are also extended to the members of the Waterflood Monitoring Program survey team -- Bill Griffiths, Dave Schmidt, Brad Adams, Terry Carpenter, Rob Dillinger a nd Dennis Hensel -- who provided the fish for the experiment; to Scott And erson for his statistical a dvi c e ; to Chuck Davis for his help in c onstruct ing the test apparatus; to Bonnie Bower for drafting the figures; and to the s t a f f s of LGL Ecological Research Associates and LGL Alaska for their help a nd encouragement. This study was funded partially by the Bureau of Land Management through i n t e rag e nc y agreement with the National Oceanic and Atmospheric Administration, as part of the Outer Continental Shelf Environmental Assessment Program. 3 ABSTRACT Ho ri zont a l - t he r ma l - g r a d i e n t apparatus of previously undescr ibed design was used to determine the temperature preference of juvenile arctic cisco, (oreganus autumnalis, as a function of acclimation temperature and acclimation-test salinity. -

New Siberian Islands Archipelago)
Detrital zircon ages and provenance of the Upper Paleozoic successions of Kotel’ny Island (New Siberian Islands archipelago) Victoria B. Ershova1,*, Andrei V. Prokopiev2, Andrei K. Khudoley1, Nikolay N. Sobolev3, and Eugeny O. Petrov3 1INSTITUTE OF EARTH SCIENCE, ST. PETERSBURG STATE UNIVERSITY, UNIVERSITETSKAYA NAB. 7/9, ST. PETERSBURG 199034, RUSSIA 2DIAMOND AND PRECIOUS METAL GEOLOGY INSTITUTE, SIBERIAN BRANCH, RUSSIAN ACADEMY OF SCIENCES, LENIN PROSPECT 39, YAKUTSK 677980, RUSSIA 3RUSSIAN GEOLOGICAL RESEARCH INSTITUTE (VSEGEI), SREDNIY PROSPECT 74, ST. PETERSBURG 199106, RUSSIA ABSTRACT Plate-tectonic models for the Paleozoic evolution of the Arctic are numerous and diverse. Our detrital zircon provenance study of Upper Paleozoic sandstones from Kotel’ny Island (New Siberian Island archipelago) provides new data on the provenance of clastic sediments and crustal affinity of the New Siberian Islands. Upper Devonian–Lower Carboniferous deposits yield detrital zircon populations that are consistent with the age of magmatic and metamorphic rocks within the Grenvillian-Sveconorwegian, Timanian, and Caledonian orogenic belts, but not with the Siberian craton. The Kolmogorov-Smirnov test reveals a strong similarity between detrital zircon populations within Devonian–Permian clastics of the New Siberian Islands, Wrangel Island (and possibly Chukotka), and the Severnaya Zemlya Archipelago. These results suggest that the New Siberian Islands, along with Wrangel Island and the Severnaya Zemlya Archipelago, were located along the northern margin of Laurentia-Baltica in the Late Devonian–Mississippian and possibly made up a single tectonic block. Detrital zircon populations from the Permian clastics record a dramatic shift to a Uralian provenance. The data and results presented here provide vital information to aid Paleozoic tectonic reconstructions of the Arctic region prior to opening of the Mesozoic oceanic basins. -

Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries
JANUARY 2020 Wholesale Market Profiles for Alaska Groundfish and FisheriesCrab Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries JANUARY 2020 JANUARY Prepared by: McDowell Group Authors and Contributions: From NOAA-NMFS’ Alaska Fisheries Science Center: Ben Fissel (PI, project oversight, project design, and editor), Brian Garber-Yonts (editor). From McDowell Group, Inc.: Jim Calvin (project oversight and editor), Dan Lesh (lead author/ analyst), Garrett Evridge (author/analyst) , Joe Jacobson (author/analyst), Paul Strickler (author/analyst). From Pacific States Marine Fisheries Commission: Bob Ryznar (project oversight and sub-contractor management), Jean Lee (data compilation and analysis) This report was produced and funded by the NOAA-NMFS’ Alaska Fisheries Science Center. Funding was awarded through a competitive contract to the Pacific States Marine Fisheries Commission and McDowell Group, Inc. The analysis was conducted during the winter of 2018 and spring of 2019, based primarily on 2017 harvest and market data. A final review by staff from NOAA-NMFS’ Alaska Fisheries Science Center was completed in June 2019 and the document was finalized in March 2016. Data throughout the report was compiled in November 2018. Revisions to source data after this time may not be reflect in this report. Typically, revisions to economic fisheries data are not substantial and data presented here accurately reflects the trends in the analyzed markets. For data sourced from NMFS and AKFIN the reader should refer to the Economic Status Report of the Groundfish Fisheries Off Alaska, 2017 (https://www.fisheries.noaa.gov/resource/data/2017-economic-status-groundfish-fisheries-alaska) and Economic Status Report of the BSAI King and Tanner Crab Fisheries Off Alaska, 2018 (https://www.fisheries.noaa. -

Identification of Larvae of Three Arctic Species of Limanda (Family Pleuronectidae)
Identification of larvae of three arctic species of Limanda (Family Pleuronectidae) Morgan S. Busby, Deborah M. Blood & Ann C. Matarese Polar Biology ISSN 0722-4060 Polar Biol DOI 10.1007/s00300-017-2153-9 1 23 Your article is protected by copyright and all rights are held exclusively by 2017. This e- offprint is for personal use only and shall not be self-archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Polar Biol DOI 10.1007/s00300-017-2153-9 ORIGINAL PAPER Identification of larvae of three arctic species of Limanda (Family Pleuronectidae) 1 1 1 Morgan S. Busby • Deborah M. Blood • Ann C. Matarese Received: 28 September 2016 / Revised: 26 June 2017 / Accepted: 27 June 2017 Ó Springer-Verlag GmbH Germany 2017 Abstract Identification of fish larvae in Arctic marine for L. proboscidea in comparison to the other two species waters is problematic as descriptions of early-life-history provide additional evidence suggesting the genus Limanda stages exist for few species. Our goal in this study is to may be paraphyletic, as has been proposed in other studies. -

Joint PINRO/IMR Report on the State of the Barents Sea Ecosystem 2006, with Expected Situation and Considerations for Management
IMR/PINRO J O S I E N I 2 R T 2007 E R E S P O R T JOINT PINRO/IMR REPORT ON THE STATE OFTHE BARENTS SEA ECOSYSTEM IN 2006 WITH EXPECTED SITUATION AND CONSIDERATIONS FOR MANAGEMENT Institute of Marine Research - IMR Polar Research Institute of Marine Fisheries and Oceanography - PINRO This report should be cited as: Stiansen, J.E and A.A. Filin (editors) Joint PINRO/IMR report on the state of the Barents Sea ecosystem 2006, with expected situation and considerations for management. IMR/PINRO Joint Report Series No. 2/2007. ISSN 1502-8828. 209 pp. Contributing authors in alphabetical order: A. Aglen, N.A. Anisimova, B. Bogstad, S. Boitsov, P. Budgell, P. Dalpadado, A.V. Dolgov, K.V. Drevetnyak, K. Drinkwater, A.A. Filin, H. Gjøsæter, A.A. Grekov, D. Howell, Å. Høines, R. Ingvaldsen, V.A. Ivshin, E. Johannesen, L.L. Jørgensen, A.L. Karsakov, J. Klungsøyr, T. Knutsen, P.A. Liubin, L.J. Naustvoll, K. Nedreaas, I.E. Manushin, M. Mauritzen, S. Mehl, N.V. Muchina, M.A. Novikov, E. Olsen, E.L. Orlova, G. Ottersen, V.K. Ozhigin, A.P. Pedchenko, N.F. Plotitsina, M. Skogen, O.V. Smirnov, K.M. Sokolov, E.K. Stenevik, J.E. Stiansen, J. Sundet, O.V. Titov, S. Tjelmeland, V.B. Zabavnikov, S.V. Ziryanov, N. Øien, B. Ådlandsvik, S. Aanes, A. Yu. Zhilin Joint PINRO/IMR report on the state of the Barents Sea ecosystem in 2006, with expected situation and considerations for management ISSUE NO.2 Figure 1.1. Illustration of the rich marine life and interactions in the Barents Sea. -

Spatial Ecology and Fisheries Interactions of Rajidae in the Uk
UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences SPATIAL ECOLOGY AND FISHERIES INTERACTIONS OF RAJIDAE IN THE UK Samantha Jane Simpson Thesis for the degree of DOCTOR OF PHILOSOPHY APRIL 2018 UNIVERSITY OF SOUTHAMPTON 1 2 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences Doctor of Philosophy FINE-SCALE SPATIAL ECOLOGY AND FISHERIES INTERACTIONS OF RAJIDAE IN UK WATERS by Samantha Jane Simpson The spatial occurrence of a species is a fundamental part of its ecology, playing a role in shaping the evolution of its life history, driving population level processes and species interactions. Within this spatial occurrence, species may show a tendency to occupy areas with particular abiotic or biotic factors, known as a habitat association. In addition some species have the capacity to select preferred habitat at a particular time and, when species are sympatric, resource partitioning can allow their coexistence and reduce competition among them. The Rajidae (skate) are cryptic benthic mesopredators, which bury in the sediment for extended periods of time with some species inhabiting turbid coastal waters in higher latitudes. Consequently, identifying skate fine-scale spatial ecology is challenging and has lacked detailed study, despite them being commercially important species in the UK, as well as being at risk of population decline due to overfishing. This research aimed to examine the fine-scale spatial occurrence, habitat selection and resource partitioning among the four skates across a coastal area off Plymouth, UK, in the western English Channel. In addition, I investigated the interaction of Rajidae with commercial fisheries to determine if interactions between species were different and whether existing management measures are effective. -

Table of Contents
Table of Contents Chapter 2. Alaska Arctic Marine Fish Inventory By Lyman K. Thorsteinson .............................................................................................................. 23 Chapter 3 Alaska Arctic Marine Fish Species By Milton S. Love, Mancy Elder, Catherine W. Mecklenburg Lyman K. Thorsteinson, and T. Anthony Mecklenburg .................................................................. 41 Pacific and Arctic Lamprey ............................................................................................................. 49 Pacific Lamprey………………………………………………………………………………….…………………………49 Arctic Lamprey…………………………………………………………………………………….……………………….55 Spotted Spiny Dogfish to Bering Cisco ……………………………………..…………………….…………………………60 Spotted Spiney Dogfish………………………………………………………………………………………………..60 Arctic Skate………………………………….……………………………………………………………………………….66 Pacific Herring……………………………….……………………………………………………………………………..70 Pond Smelt……………………………………….………………………………………………………………………….78 Pacific Capelin…………………………….………………………………………………………………………………..83 Arctic Smelt………………………………………………………………………………………………………………….91 Chapter 2. Alaska Arctic Marine Fish Inventory By Lyman K. Thorsteinson1 Abstract Introduction Several other marine fishery investigations, including A large number of Arctic fisheries studies were efforts for Arctic data recovery and regional analyses of range started following the publication of the Fishes of Alaska extensions, were ongoing concurrent to this study. These (Mecklenburg and others, 2002). Although the results of included -

INVERTEBRATE SPECIES in the EASTERN BERING SEA By
Effects of areas closed to bottom trawling on fish and invertebrate species in the eastern Bering Sea Item Type Thesis Authors Frazier, Christine Ann Download date 01/10/2021 18:30:05 Link to Item http://hdl.handle.net/11122/5018 e f f e c t s o f a r e a s c l o s e d t o b o t t o m t r a w l in g o n fish a n d INVERTEBRATE SPECIES IN THE EASTERN BERING SEA By Christine Ann Frazier RECOMMENDED: — . /Vj Advisory Committee Chair Program Head / \ \ APPROVED: M--- —— [)\ Dean, School of Fisheries and Ocean Sciences • ~7/ . <-/ / f a Dean of the Graduate Sch6oI EFFECTS OF AREAS CLOSED TO BOTTOM TRAWLING ON FISH AND INVERTEBRATE SPECIES IN THE EASTERN BERING SEA A THESIS Presented to the Faculty of the University of Alaska Fairbanks in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE 6 By Christine Ann Frazier, B.A. Fairbanks, Alaska December 2003 UNIVERSITY OF ALASKA FAIRBANKS ABSTRACT The Bering Sea is a productive ecosystem with some of the most important fisheries in the United States. Constant commercial fishing for groundfish has occurred since the 1960s. The implementation of areas closed to bottom trawling to protect critical habitat for fish or crabs resulted in successful management of these fisheries. The efficacy of these closures on non-target species is unknown. This study determined if differences in abundance, biomass, diversity and evenness of dominant fish and invertebrate species occur among areas open and closed to bottom trawling in the eastern Bering Sea between 1996 and 2000.