Namibian Cheetah & Lion PHVA Final Report.7.0.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019 Annual Report
ANNUAL REPORT 2019 Potter Park Zoo Phone: 517.483.4222 1301 S. Pennsylvania Ave. Fax: 517.316.3894 Lansing, MI 48912 www.potterparkzoo.org TABLE OF CONTENTS 2 Letter from the Directors 4 Our Animals During 2019 191,406 people visited Potter Park Zoo. 5 Conservation 6 Veterinary Care 8 Volunteers 9 Education Programs 2019 Events INCENTIVE DAYS 2019 10 Number of free or reduced admissions 11 Members Mother's Day 834 Father's Day 164 Be a Tourist in Your Own Town 2,369 Zoo Days 7,179 12 Marketing & Communications College Day 122 Ingham County Free Day 159 Ingham County Free Monday Mornings 8,208 13 Zoo Team 14 Donor Recognition 16 Zoo Board & Finances We have been accredited by the AZA for over 30 years. We are proud to continue to meet and exceed 17 Potter Park Zoological Society the standards set by AZA in animal welfare, veterinary care, conservation, education, guests services and more. We pride ourselves on being an enriching educational resource for our community. 31 1 A letter from Cynthia Wagner, Potter Park Zoo Director and Amy L. Morris-Hall, Potter Park Zoological Society Director With the hard work and dedication of our staff and volunteers, Potter Park Zoo experienced many successes in 2019. Through our passion and expertise in animal care, conservation and education, we continued to fulfill our mission to inspire conservation of animals and the natural world. We knocked down barriers and evolved our education programs to not only raise the bar for ourselves, but for other institutions around the country. -

RFP Number and Title: P2013000012, "Biopark Asian Jungle Ropes & Zip Line Course"
` City of Albuquerque Request for Proposals Solicitation Number: P2013000012 BioPark Asian Jungle Ropes & Zip Line Course Deadline for Receipt of Offers October 26, 2012: 4:00 p.m. (Mountain Time) The City eProcurement System will not allow proposals to be submitted after this date and time. Pre-proposal Conference: October 9, 2012 2:30 PM Albuquerque BioPark 903 Tenth Street Albuquerque, NM 87102 Library City of Albuquerque P2013000012, " BioPark Asian Jungle Ropes & Zip Line Course” 1 Department of Finance and Administrative Services Purchasing Division TABLE OF CONTENTS Page Introduction 3 Part 1 Instructions to Offerors 4 Part 2 Proposal Format 13 Part 3 Scope of Services 15 Part 4 Evaluation of Offers 16 Part 5 Local Preference Instructions 18 Local Preference Form 19 Part 6 Draft Agreement 22 Appendix A Revenue Proposal Forms 28 Appendix B Bond Forms 29 P2013000012, " BioPark Asian Jungle Ropes & Zip Line Course” 2 INTRODUCTION Purpose and Intent The Albuquerque Biological Park (BioPark) is soliciting proposals from qualified firms interested in providing an Asian Jungle Experience using a combination of rope courses and zip lines on a 2 acre parcel of land located at the Rio Grande Zoo, 903 10th St. Albuquerque NM 87102. The Albuquerque Biological Park is an accredited facility through the Association of Zoos and Aquariums (AZA) and seeks to maintain and continually improve upon the care and management of the animal and plant collection as well as providing an important recreational and educational venue to the citizens and visitors of the Albuquerque region. Background and General Information 1. The Albuquerque Biological Park is owned and operated by the City of Albuquerque. -

General Vertical Files Anderson Reading Room Center for Southwest Research Zimmerman Library
“A” – biographical Abiquiu, NM GUIDE TO THE GENERAL VERTICAL FILES ANDERSON READING ROOM CENTER FOR SOUTHWEST RESEARCH ZIMMERMAN LIBRARY (See UNM Archives Vertical Files http://rmoa.unm.edu/docviewer.php?docId=nmuunmverticalfiles.xml) FOLDER HEADINGS “A” – biographical Alpha folders contain clippings about various misc. individuals, artists, writers, etc, whose names begin with “A.” Alpha folders exist for most letters of the alphabet. Abbey, Edward – author Abeita, Jim – artist – Navajo Abell, Bertha M. – first Anglo born near Albuquerque Abeyta / Abeita – biographical information of people with this surname Abeyta, Tony – painter - Navajo Abiquiu, NM – General – Catholic – Christ in the Desert Monastery – Dam and Reservoir Abo Pass - history. See also Salinas National Monument Abousleman – biographical information of people with this surname Afghanistan War – NM – See also Iraq War Abousleman – biographical information of people with this surname Abrams, Jonathan – art collector Abreu, Margaret Silva – author: Hispanic, folklore, foods Abruzzo, Ben – balloonist. See also Ballooning, Albuquerque Balloon Fiesta Acequias – ditches (canoas, ground wáter, surface wáter, puming, water rights (See also Land Grants; Rio Grande Valley; Water; and Santa Fe - Acequia Madre) Acequias – Albuquerque, map 2005-2006 – ditch system in city Acequias – Colorado (San Luis) Ackerman, Mae N. – Masonic leader Acoma Pueblo - Sky City. See also Indian gaming. See also Pueblos – General; and Onate, Juan de Acuff, Mark – newspaper editor – NM Independent and -

Publication of the Utica Zoological Society Volume 4 Number 3
Publication of the Utica Zoological Society May/June 1996 Volume 4 Number 3 ~..,.- Spring has arrived ...~nally! It~~--~,._ sensitive. The babies have tender was a long, cold wmter, bu -::: ~-. v~ ~ . ink skin and are easily sunburned underneath several ~eet of snow, · - ~· - , e;' ' uring their first few weeks of life. plants and arumals are To prevent this mom will lead her beginning to feel the change of the whale . family, are usually baby to a mud hoie so they can cover seasons.For many species of wildlife, h~lthy and robust ~t birth. Th~y themselves with~ pr?,tective co~ting of spring is the time for giving birth. The qm~kly adapt t? their new aq~IC mud, sort of a rhino sunscreen . ycle of life is quite different for envrronment, With mother watching When they are born, African • mammals, birds, fish, amphibians and them very closely. The mother colobus monkeys are covered with reptiles. All mammals (with the guides her calfs movements, and curly white fur! The infants are passed exception of a few oddities such as the some mothers have even been back and forth among the troop's duck-billed platypus and the spiny observed disciplining the calves females, and it is believed that the all- ~teater) give birth to l.iv~ young, while when they misbehave! The calf white fur of the babies encourages this birds, fish and amp~tbians lay eg~s . nurses within an hour after it is behavior. Because they look different Snakes are an exception: some spectes . · b'rth t . born, and as often as four times from the adults, who are black With gtve 1 o 1IVe young, some 1ay eggs, . -
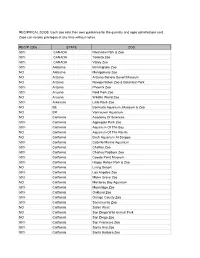
2006 Reciprocal List
RECIPRICAL ZOOS. Each zoo sets their own guidelines for the quantity and ages admitted per card. Zoos can revoke privileges at any time without notice. RECIP 2006 STATE ZOO 50% CANADA Riverview Park & Zoo 50% CANADA Toronto Zoo 50% CANADA Valley Zoo 50% Alabama Birmingham Zoo NO Alabama Montgomery Zoo NO Arizona Arizona-Sonora Desert Museum NO Arizona Navajo Nation Zoo & Botanical Park 50% Arizona Phoenix Zoo 50% Arizona Reid Park Zoo NO Arizona Wildlife World Zoo 50% Arkansas Little Rock Zoo NO BE Bermuda Aquarium, Museum & Zoo NO BR Vancouver Aquarium NO California Academy Of Sciences 50% California Applegate Park Zoo 50% California Aquarium Of The Bay NO California Aquarium Of The Pacific NO California Birch Aquarium At Scripps 50% California Cabrillo Marine Aquarium 50% California Chaffee Zoo 50% California Charles Paddock Zoo 50% California Coyote Point Museum 50% California Happy Hollow Park & Zoo NO California Living Desert 50% California Los Angeles Zoo 50% California Micke Grove Zoo NO California Monterey Bay Aquarium 50% California Moonridge Zoo 50% California Oakland Zoo 50% California Orange County Zoo 50% California Sacramento Zoo NO California Safari West NO California San Diego Wild Animal Park NO California San Diego Zoo 50% California San Francisco Zoo 50% California Santa Ana Zoo 50% California Santa Barbara Zoo NO California Seaworld San Diego 50% California Sequoia Park Zoo NO California Six Flags Marine World NO California Steinhart Aquarium NO CANADA Calgary Zoo 50% Colorado Butterfly Pavilion NO Colorado Cheyenne -

Kirtland Inn Guestbook
Dear Guest, Welcome to the Kirtland Inn, setting the standard for the USAF! On behalf of the staff, we sincerely hope your stay is pleasant and comfortable. Kirtland Inn and Kirtland Air Force Base are rich in history and tradition. Please take time out of your busy schedule to enjoy the sights and the surrounding area. We strive to provide outstanding guest service and accommodations to all of our patrons. Should you need anything special or if you have inadvertently forgotten a travel item, please ask any staff member for assistance. We will take care of your request as quickly as possible. If you need further information about Kirtland Air Force Base and the surrounding community, just ask. Continuous improvement is our goal. We welcome any comments or recommendations that you may have. Please complete the comment card located in your room or at the Reception Desks to let us know how we did. You can reach me at 846-9663 or any of our Guest Service Representatives by dialing 0. Again, welcome to Kirtland AFB and the Kirtland Inn! Steve Holland, CHA General Manager Email: [email protected] The appearance of local business names does NOT imply federal endorsements. All information to include addresses and telephone numbers are subject to change. Please call the business to confirm their operation hours. GENERAL INFORMATION This information is made available as a public service and does not imply Air Force endorsement of the company’s products or services. CALL 853-9111 FOR ANY ON BASE EMERCENCY Kirtland AFB Gate Information Gate Phone Eubank Gate 846-6231 Gibson Gate 846-7240 Maxwell Gate 846-7491 Truman Gate 846-7509 Wyoming Gate 846-6118 Receiving Mail During Your Stay Guests who require mail service while staying at the Kirtland Inn should use the base general delivery address: Your Name General Delivery 2050 2nd St SE Kirtland Air Force Base, NM 87117 *Upon arrival to Kirtland AFB, individuals utilizing general delivery mail service must come by the Postal Service Center, building 20204, to fill out a locator card with your new address. -

Population Analysis & Breeding and Transfer Plan Cheetah
Draft for Institutional Representative Review – Please Respond by 3 March 2017 Population Analysis & Breeding and Transfer Plan Cheetah (Acinonyx jubatus) AZA Species Survival Plan® Yellow Program AZA Species Survival Plan® Coordinator Adrienne Crosier, PhD, Smithsonian’s Conservation Biology Institute – Center for Species Survival, ([email protected]) & AZA Studbook Keeper Erin Moloney, Busch Gardens, ([email protected]) AZA Population Advisor John Andrews, Population Management Center ([email protected]) 1 February 2017 Draft for Institutional Representative Review – Please Respond by 3 March 2017 Executive Summary 2017 Species Survival Plan® for the Cheetah (Acinonyx jubatus) The Cheetah SSP population currently consists of 315 cats (151 males, 164 females) at 59 facilities (55 AZA, 4 non- AZA) as of 18 January 2017. Currently, there are thirteen cheetahs (9.4) held at non-AZA facilities. The target population size set by the Felid Taxon Advisory Group is 300 in the 2009 Regional Collection Plan. The population currently qualifies as a Yellow SSP Program. Current gene diversity for the managed population is 97.27% and is equivalent to the genetic diversity of a population descended from approximately 18 founders (FGE = 18.34). The standard genetic goal for AZA managed populations is to maintain 90% gene diversity for 100 years. When gene diversity falls below 90% (and average inbreeding increases), it is expected that reproduction will be increasingly compromised by, among other factors, lower birth weights, smaller litter sizes, and greater neonatal mortality. Under potential conditions, with a growth rate of 0% (lambda = 1.00), gene diversity can be maintained at or above 90% gene diversity for at least 50 years. -

Warren J. Rauhe
VITAE Warren J. Rauhe Office address: School of Planning, Design and Construction 313 Human Ecology Michigan State University East Lansing, Michigan 48824-1030 [email protected] (517) 432 0704 [email protected] EDUCATION M.A. 1982, Central Michigan University, Business Administration M.L.A. 1973, University of Illinois. Thesis: "Definition, Description and Location of Natural Areas on the Landscape" B.L.A. 1970, S.U.N.Y. College of Environmental Science and Forestry, School of Landscape Architecture and Syracuse University (cum laude) Management of Design and Planning Firms--Short Course, Harvard University Graduate School of Design LICENSING AND MEMBERSHIPS Licensed Landscape Architect in Michigan, License #750 Former member, State of Michigan Landscape Architects Registration Board National Council of Landscape Architectural Registration Boards, Certification #88 (inactive) Member, American Society of Landscape Architects (ASLA) Member, Council of Educators in Landscape Architecture (CELA) Honorary Member, "YUTYAM," Research and Design Center for Touristic Development, Yaldiz University, Istanbul, Turkey HONORS AND AWARDS Special Tributes State of Michigan Eighty-Sixth Legislature Special Tribute to W.J. Rauhe and students for "improvement in the landscape architecture, planning, and design work in Bay County, Michigan." U.S. Environmental Protection Agency, Tribute of Appreciation to MSU, the LAP, and W.J. Rauhe "for directing the development of an outstanding design project for the proposed USEPA site, Bay City, Michigan." 1 Design Awards 2004 First Place, National Competition DaimlerChrysler New World Engine Plant Landscape, Dundee, Michigan. 2004 "Sculpting Movement: Restoring Spirit." By 26 volunteers from MSU Landscape Architecture and Horticulture. Advisor. 1992 ASLA/Michigan Chapter Merit Award. "CT Enterprises: Sequential Mining and Reclamation Program." (Co-Designers: Anthony M. -

Rio Grande Heritage Farm Education Guide
Rio Grande Heritage Farm Education Guide Grades 4-5 Contents General Biopark Information Biopark Group Admission Information School Lunch Order Form General Teacher Tips Chaperone Guide Teacher Background Information Pre-Visit Activities Field Trip Worksheet Post-Visit Activities Student Handouts Student Handout Answer Sheets GENERAL BIOPARK INFORMATION Facilities The Albuquerque BioPark is a gateway to the mystery and diversity of living organisms. Core facilities include: Albuquerque Aquarium, Rio Grande Botanic Garden, Rio Grande Zoo and Tingley Beach. The BioPark has an estimated 6,000 animals, 11,000 plants, 300 staff and 300 year-round volunteers. The Director of the Albuquerque BioPark is Ray Darnell. The Albuquerque Aquarium is a great place to explore aquatic environments by tracing a drop of water from the headwaters of the Rio Grande in southern Colorado to the Gulf of Mexico and out to the oceans of the world. Current exhibits highlight fish of the Rio Grande at Central Bridge, moray eels, invertebrates, coral reefs, floating jellies, sea turtles and lots of toothy sharks. Further construction will develop tanks that highlight species that live in the Pacific Ocean. (Location 2601 Central NW) The Rio Grande Botanic Garden celebrates the miracle of photosynthesis in living color every day. Our state-of-the-art glass conservatories feature plants native to Mediterranean climate zones and xeric plants from North American deserts and other arid regions of the world. A trio of formal walled gardens illustrates Old World design in fountains, tile, herbs and roses. El Jardin de la Curandera is an ethnobotanic exhibit with a beautiful bronze sculpture, and the Children’s Fantasy Garden has giant fun for kids of all ages. -

Michigan Karner Blue Butterfly HCP
DRAFT MICHIGAN KARNER BLUE BUTTERFLY HABITAT CONSERVATION PLAN Photograph by Jennifer Kleitch ATU F N RA O L T R Printed by Authority of: P.A. 451 of 1994 N E Michigan Department of Natural Resources E S Total Number of Copies Printed: .........XX M O T U Cost per Copy:.................................$XXX R R Wildlife Division Report No. _____ C A P Total Cost: ......................................$XXX DNR E E S D _____ 2007 Michigan Department of Natural Resources M ICHIG AN ICXXXXX (XXXXXX) DRAFT – November 2, 2007 2 DRAFT MICHIGAN KARNER BLUE BUTTERFLY HABITAT CONSERVATION PLAN Prepared by: Michigan Department of Natural Resources Wildlife Division Stevens T. Mason Building P.O. Box 30180 Lansing, MI 48909 Submitted to: U.S. Fish and Wildlife Service East Lansing Field Office 2651 Coolidge Road, Suite 101 East Lansing, Michigan 48823 November 2, 2007 DRAFT – November 2, 2007 3 ACKNOWLEDGMENTS The Michigan Department of Natural Resources appreciates the valuable contributions made by many agencies, organizations and individuals during the development of this plan. In particular, we thank the U.S. Fish and Wildlife Service for providing funding and technical support. We also thank the members of the Karner Blue Butterfly Working Group and the Karner Blue Butterfly Management Partners Workgroup, who shared important perspectives and expertise during their meetings and document reviews. Finally, we thank the members of the public who helped shape the content of this plan by offering input during public meetings and public- comment periods. A contribution of the Cooperative Endangered Species Conservation Fund Grants Program, Michigan Project E-3-HP Equal Rights for Natural Resource Users The Michigan Department of Natural Resources (MDNR) provides equal opportunities for employment and access to Michigan’s natural resources. -

AZVT Presentation Title History
AZVT Presentation Title History Titles of previously presented posters, presentations, round table discussions, and wet labs from past years - to reference for future presentations. Please review this information when deciding on your topic. Some topics are rather popular and often brought up for presentation. In the interest of keeping things fresh and sharing new information, the Editorial Committee will evaluate the submitted topic and evaluate whether or not it has been presented too much in the recent past. Please keep in mind that if a topic has been covered frequently in the recent past, you may be asked to rethink your subject matter or asked to wait for another year. 40th Annual Meeting – 3-6 October, 2020 Virtual Conference – Run The World PRESENTATIONS Note: (P) denotes full paper; (A) denotes abstract only Shocking! Electric Eel (Electrophorus electricus) Restraint, Anesthesia, Anatomy, and (P) McMurrer Water Quality Challenges Elasmobranch Anesthesia: An Overview (P) Barron Cliffside Phlebotomy, Cryo-Shippers and More: A Crash Course in Field Research (A) Cambell with Gentoo (Pygoscelis papua) and Southern Rockhopper (Eudyptes chrysocome) Penguins in the Falkland Islands Giant Manta Ray (Manta birostris) Exams: Sample Collection and Processing (P) Kliesrath Diagnostics and Treatment Protocols in Response to an Acute Mortality Event in Sea (P) Dunleavy Urchins (Strongylocentrotus spp., Mesocentrotus franciscanus) Lactate Meter: An Underutilized Tool for Assessment of Hypoperfusion (P) Merkel Making a Safe Zoo Safer (A) McNally -

Middle Rio Grande Microbial Source Tracking Assessment Report
Middle Rio Grande Microbial Source Tracking Assessment Report Prepared for New Mexico Environment Department Albuquerque Metropolitan Arroyo Flood Control Authority Bernalillo County Prepared by Parsons Water & Infrastructure Inc. 8000 Centre Park Drive, Suite 200 Austin, TX 78754 October 2005 Middle Rio Grande Microbial Source Tracking Assessment Report Prepared for New Mexico Environment Department Albuquerque Metropolitan Arroyo Flood Control Authority Bernalillo County Prepared by Parsons Water & Infrastructure Inc. 8000 Centre Park Drive, Suite 200 Austin, TX 78754 October 2005 This study was funded by the New Mexico Environment Department’s Surface Water Quality Bureau, Bernalillo County, and the Albuquerque Metropolitan Arroyo Flood Control Authority. Middle Rio Grande Microbial Source Tracking Executive Summary EXECUTIVE SUMMARY This Middle Rio Grande (MRG) Microbial Source Tracking Project was funded by the New Mexico Environmental Department, Albuquerque Metropolitan Arroyo Flood Control Authority, and Bernalillo County. The objective of this project was to identify specific sources of fecal coliform causing high levels of bacteria in the MRG. The three agencies recognized that this type of data was essential to provide water quality program managers and stakeholders the information necessary to target solutions for reducing fecal coliform concentrations/loadings within the surface waters of the study area. The study area lies within the MRG between Angostura Diversion Dam in southeastern Sandoval County to the Isleta Diversion Dam, at the northern border of Isleta Pueblo, a distance of approximately 42 river miles (Figure 2.1). To identify specific sources of fecal coliform by subwatershed, this project involved several steps: • Execution of a sanitary survey or reconnaissance tour of the watershed and a compilation of available data and literature to identify potential contributing sources of fecal bacteria to be considered.