Effect of Hyperbaric Oxygen Therapy on the Development of Airway Stenosis in Patients with Severe Airway Exudative Plaques Early After Lung Transplantation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019 Client Experience Summit Learn. Share. Succeed. 3M HIS Nosology Teams
2019 Client Experience Summit Learn. Share. Succeed. Nosology Nuggets Michele Taylor RHIT, CCS Rauna Gale RHIA 2019 Client Experience Summit Learn. Share. Succeed. 3M HIS Nosology Teams Nosology CAC Nosology Support Nosology Development Team Development Team Teams • 18 team members • 28 team members • 35 team members • RHIA, RHIT, • RHIA, RHIT, CCS, • RHIA, RHIT, CCS, CCS, CPC, CPC, RN, CDIP CCDS CPC-H, CDIP, IR CIRCC High-Volume Nosology Topics - Agenda ➢ Sepsis Sequencing ➢ Rehabilitation ➢ Bronchoscopy PCS ➢ CHF grouping ➢ NCCI and Other Edits with CMS website tips and tricks ➢ Separate Procedure Designation ➢ Podiatry ➢ LINX Sepsis Sequencing Sepsis Sequencing • If the reason for admission is both sepsis or severe sepsis and a localized infection, such as pneumonia or cellulitis, a code(s) for the underlying systemic infection (A41.9) should be assigned first and the code for the localized infection should be assigned as a secondary diagnosis. • If sepsis or severe sepsis is documented as associated with a noninfectious condition, such as a burn or serious injury, and this condition meets the definition for principal diagnosis, the code for the noninfectious condition should be sequenced first, followed by the code for the resulting infection. • If the infection (sepsis) meets the definition of principal diagnosis, it should be sequenced before the non-infectious condition. When both the associated non- infectious condition and the infection meet the definition of principal diagnosis, either may be assigned as principal diagnosis. Source: ICD-10-CM Official Guidelines for Coding and Reporting FY 2019 Page 25-27 Scenario: Patient is admitted with left diabetic foot ulcer with cellulitis and gangrene. -

Diagnostic Direct Laryngoscopy, Bronchoscopy & Esophagoscopy
Post-Operative Instruction Sheet Diagnostic Direct Laryngoscopy, Bronchoscopy & Esophagoscopy Direct Laryngoscopy: Examination of the voice box or larynx (pronounced “lair-inks”) under general anesthesia. An instrument called a laryngoscope is carefully placed into the mouth and used to visualize the larynx and surrounding structures. Bronchoscopy: Examination of the windpipe below the voice box in the neck and chest under general anesthesia. A long narrow telescope is passed through the larynx and used to carefully inspect the structures of the trachea and bronchi. Esophagoscopy: Examination of the swallowing pipe in the neck and chest under general anesthesia. An instrument called an esophagoscope is passed into the esophagus (just behind the larynx and trachea) and used to visualize the mucus membranes and surrounding structures of the esophagus. Frequently a small biopsy is taken to evaluate for signs of esophageal inflammation (esophagitis). What to Expect: Diagnostic airway endoscopy procedures generally take about 45 minutes to complete. Usually the procedure is well-tolerated and the child is back-to-normal the next day. Mild throat or tongue discomfort may persist for a few days after the procedure and is usually well-controlled with over-the-counter acetaminophen (Tylenol) or ibuprofen (Motrin). Warning Signs: Contact the office immediately at (603) 650-4399 if any of the following develop: • Worsening harsh, high-pitched noisy-breathing (stridor) • Labored breathing with chest retractions or flaring of the nostrils • Bluish discoloration of the lips or fingernails (cyanosis) • Persistent fever above 102°F that does not respond to Tylenol or Motrin • Excessive coughing or respiratory distress during feeding • Coughing or throwing up bright red blood • Excessive drowsiness or unresponsiveness Diet: Resume baseline diet (no special postoperative diet restrictions). -

Tracheal Wash Vs BAL 2
TRACHEAL WASHES IN DOGS AND CATS: WHY, WHAT, WHEN, AND HOW Eleanor C. Hawkins, DVM, Dipl ACVIM (SAIM) Professor, Small Animal Internal Medicine North Carolina State University Raleigh, North Carolina, USA 1. Introduction: Tracheal Wash vs BAL 2. Focus on Tracheal Wash TRACHEAL WASH vs BAL 1 TRACHEAL WASH vs BAL • Exudate from airways and alveoli • Samples all alveoli dependent on to the trachea via mucociliary the bronchus where scope or clearance +/‐ cough. catheter is lodged. • Good representation for most • Primarily a deep lung sample: diffuse bronchial disease and small airways, alveoli, and aspiration or bronchopneumonia. sometimes the interstitium. TRACHEAL WASH vs BAL TRACHEAL WASH vs BAL 2 DEFINITIONS – Slippery slope • TW becomes BAL • Catheter into small airways • Relatively large volumes of fluid used • BAL becomes TW • Single bolus • Relatively small volume Regardless of method • Tracheal wash (TW) and bronchoalveolar lavage (BAL) result in sufficient material for: Cytology Cultures PCR Flow cytometry Special stains / markers Cell function testing • BAL: greater volume, more cells than TW TW and BAL Cytology • Similar benefits › Less invasive than getting tissue 3 TW and BAL Cytology • Similar limitations › No architecture › Cells must exfoliate • E.g. not pulmonary fibrosis • E.g. not sarcoma › Organisms must be present in large numbers › Secondary processes must not “hide” primary • Infection vs non‐infectious disease • Inflammation vs neoplasia TW and BAL • MOST USEFUL FOR • Ruling IN infectious disease • Ruling IN neoplasia -

A Clinical Prediction Rule for Pulmonary Complications After Thoracic Surgery for Primary Lung Cancer
A Clinical Prediction Rule for Pulmonary Complications After Thoracic Surgery for Primary Lung Cancer David Amar, MD,* Daisy Munoz, MD,* Weiji Shi, MS,† Hao Zhang, MD,* and Howard T. Thaler, PhD† BACKGROUND: There is controversy surrounding the value of the predicted postoperative diffusing capacity of lung for carbon monoxide (DLCOppo) in comparison to the forced expired volume in 1 s for prediction of pulmonary complications (PCs) after thoracic surgery. METHODS: Using a prospective database, we performed an analysis of 956 patients who had resection for lung cancer at a single institution. PC was defined as the occurrence of any of the following: atelectasis, pneumonia, pulmonary embolism, respiratory failure, and need for supplemental oxygen at hospital discharge. RESULTS: PCs occurred in 121 of 956 patients (12.7%). Preoperative chemotherapy (odds ratio 1.64, 95% confidence interval 1.06–2.55, P ϭ 0.02, point score 2) and a lower DLCOppo (odds ratio per each 5% decrement 1.13, 95% confidence interval 1.06–1.19, P Ͻ 0.0001, point score 1 per each 5% decrement of DLCOppo less than 100%) were independent risk factors for PCs. We defined 3 overall risk categories for PCs: low Յ10 points, 39 of 448 patients (9%); intermediate 11–13 points, 37 of 256 patients (14%); and high Ն14 points, 42 of 159 patients (26%). The median (range) length of hospital stay was significantly greater for patients who developed PCs than for those who did not: 12 (3–113) days vs 6 (2–39) days, P Ͻ 0.0001, respectively. Similarly, 30-day mortality was significantly more frequent for patients who developed PCs than for those who did not: 16 of 121 (13.2%) vs 6 of 835 (0.7%), P Ͻ 0.0001. -

Interpretation of Bronchoalveolar Lavage Fluid Cytology
Interpretation of bronchoalveolar lavage fluid cytology Contents Editor Marjolein Drent, MD, PhD Maastricht, The Netherlands Preface e-mail : [email protected] Foreword Contributors Introduction Prof. Robert Baughman, MD, PhD Cincinnati, Ohio, USA e-mail : [email protected] [email protected] History Prof. Ulrich Costabel, MD, PhD Essen, Germany e-mail: [email protected] Bronchoalveolar lavage Jan A. Jacobs, MD, PhD Text with illustrations Maastricht, The Netherlands e-mail: [email protected] Interactive predicting model Rob J.S. Lamers, MD, PhD Predicting program Heerlen, The Netherlands Software to evaluate BALF analysis e-mail: [email protected] Computer program using BALF Variables: a new release Paul G.H. Mulder, MSc, PhD Rotterdam, The Netherlands e-mail: [email protected] Prof. Herbert Y. Reynolds, MD, PhD Hershey, Pennsylvania, USA Glossary of abbreviations e-mail: [email protected] Acknowledgements Prof. Sjoerd Sc. Wagenaar, MD, PhD Amsterdam, The Netherlands e-mail: [email protected] Interpretation of BALF cytology Preface Bronchoalveolar lavage (BAL) explores large areas of the alveolar compartment providing cells as well as non-cellular constituents from the lower respiratory tract. It opens a window to the lung. Alterations in BAL fluid and cells reflect pathological changes in the lung parenchyma. The BAL procedure was developed as a research tool. Meanwhile its usefulness, also for clinical applications, has been appreciated worldwide in diagnostic work-up of infectious and non-infectious interstitial lung diseases. Moreover, BAL has several advantages over biopsy procedures. It is a safe, easily performed, minimally invasive, and well tolerated procedure. In this respect, when the clinician decides that a BAL might be helpful to provide diagnostic material, it is mandatory to consider the provided information obtained from BAL fluid analysis carefully and to have reliable diagnostic criteria. -

The Shelf in Every Comprehensive Hyperbaric Facility. Michael H
BOOKS,SOFTWARE, AND OTHER MEDIA the shelf in every comprehensive hyperbaric new and to improve existing protocols. This test for readiness with rapid-shallow- facility. collection of protocols was developed by breathing index, exercise, evaluate progress, the University of California’s Respiratory and report information to clinicians) proto- Michael H Bennett MD FANZCA Care Services Department, and is published col with an addendum for synchronized Department of Diving and by Daedalus Enterprises. The CD-ROM intermittent mandatory ventilation with Hyperbaric Medicine contains the patient-driven-protocol manual pressure support (SIMV/PS); STEER for Prince of Wales Hospital in an Abode Acrobat PDF file, and 25 pa- cardiothoracic service postoperative (day 1) and tient-driven-protocol algorithms in Mi- open-heart surgery; and metered-dose- Faculty of Medicine crosoft Visio format, which allow the reader inhaler protocol for ventilated patients. The University of New South Wales to print high-quality color versions of the protocols in Part II follow the same format Sydney, Australia protocols. Macintosh users may have diffi- as those in Part I. Because of the depth of culty viewing the files; Visio is not avail- each protocol, there is considerably more The author has disclosed no conflicts of interest. able for Macintosh computers, so a third- explanation given in the overview sections. party program is needed to view them. On Part III contains 2 pediatric protocols: Respiratory Care Patient-Driven Proto- my computer I easily accessed and printed metered-dose-inhaler protocol, and a wean- cols, 3rd edition. University of California the PDF file. ing protocol. San Diego, Respiratory Services. -

How Is Pulmonary Fibrosis Diagnosed?
How Is Pulmonary Fibrosis Diagnosed? Pulmonary fibrosis (PF) may be difficult to diagnose as the symptoms of PF are similar to other lung diseases. There are many different types of PF. If your doctor suspects you might have PF, it is important to see a specialist to confirm your diagnosis. This will help ensure you are treated for the exact disease you have. Health History and Exam Your doctor will perform a physical exam and listen to your lungs. • If your doctor hears a crackling sound when listening to your lungs, that is a sign you might have PF. • It is also important for your doctor to gather detailed information about your health. ⚪ This includes any family history of lung disease, any hazardous materials you may have been exposed to in your lifetime and any diseases you’ve been treated for in the past. Imaging Tests Tests like chest X-rays and CT scans can help your doctor look at your lungs to see if there is any scarring. • Many people with PF actually have normal chest X-rays in the early stages of the disease. • A high-resolution computed tomography scan, or HRCT scan, is an X-ray that provides sharper and more detailed pictures than a standard chest X-ray and is an important component of diagnosing PF. • Your doctor may also perform an echocardiogram (ECHO). ⚪ This test uses sound waves to look at your heart function. ⚪ Doctors use this test to detect pulmonary hypertension, a condition that can accompany PF, or abnormal heart function. Lung Function Tests There are several ways to test how well your lungs are working. -

Airway Versus Transbronchial Biopsy and BAL in Lung Transplant Recipients: Different but Complementary
Eur Respir J 1997; 10: 2876–2880 Copyright ERS Journals Ltd 1997 DOI: 10.1183/09031936.97.10122876 European Respiratory Journal Printed in UK - all rights reserved ISSN 0903 - 1936 Airway versus transbronchial biopsy and BAL in lung transplant recipients: different but complementary C. Ward, G.I. Snell, B. Orsida, L. Zheng, T.J. Williams, E.H. Walters Airway versus transbronchial biopsy and BAL in lung transplant recipients: different Dept of Respiratory Medicine, Alfred but complementary. C. Ward, G.I. Snell, B. Orsida, L. Zheng, T.J. Williams, E.H. Walters. Hospital and Monash University Medical ©ERS Journals Ltd 1997. School, Melbourne, Australia. ABSTRACT: Lung transplantation is now an established therapeutic intervention Correspondence: E.H Walters for end-stage cardiopulmonary disease in humans. Chronic rejection, in the form Dept of Respiratory Medicine of bronchiolitis obliterans syndrome (BOS), remains the commonest cause of mor- Alfred Hospital bidity and mortality in those surviving more than 3 months. The pathology of BOS Prahran involves airway changes. We have evaluated the potential for endobronchial biop- Melbourne sies (EBB) to complement existing sampling methods used in allograft monitoring Victoria 3181 and have compared the results of EBB findings with those of bronchoalveolar Australia lavage (BAL) and transbronchial biopsy (TBB) in 18 clinically stable patients. Keywords: Bronchoalveolar lavage We found that all the EBB had inflammatory cells present but that only five endobronchial biopsy TBB specimens had evidence of inflammation, with airway material being present lung transplant in 78% of the TBB. Paired BAL and EBB yielded different results, with no cor- transbronchial biopsy relations between total macrophages, lymphocytes, CD4+ cells or CD8+ cells. -
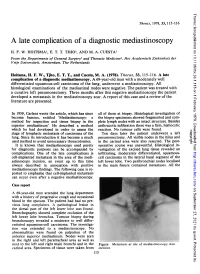
A Late Complication of a Diagnostic Mediastinoscopy
Thorax: first published as 10.1136/thx.33.1.115 on 1 February 1978. Downloaded from Thorax, 1978, 33, 115-116 A late complication of a diagnostic mediastinoscopy H. F. W. HOITSMA1, E. T. T. TJHO2, AND M. A. CUESTA' From the Departments of General Surgery' and Thoracic Medicine2, Het Academisch Ziekenhuis der Vrije Universiteit, Amsterdam, The Netherlands Hoitsma, H. F. W., Tjho, E. T. T., and Cuesta, M. A. (1978). Thorax, 33, 115-116. A late complication of a diagnostic mediastinoscopy. A 69-year-old man with a moderately well differentiated squamous-cell carcinoma of the lung, underwent a mediastinoscopy. All histological examinations of the mediastinal nodes were negative. The patient was treated with a curative left pneumonectomy. Three months after this negative mediastinoscopy the patient developed a metastasis in the mediastinoscopy scar. A report of this case and a review of the literature are presented. In 1959, Carlens wrote the article, which has since all of them at biopsy. Histological investigation of become famous, entitled 'Mediastinoscopy: a the biopsy specimens showed fragmented and com- method for inspection and tissue biopsy in the plete lymph nodes with an intact structure. Besides superior mediastinum'. He described a method anthracotic infiltration there was a firm, histiocytic which he had developed in order to assess the reaction. No tumour cells were found. copyright. stage of lymphatic metastasis of carcinoma of the Ten days later the patient underwent a left lung. Since its introduction it has become a much pneumonectomy. All visible nodes in the hilus and used method to avoid unnecessary thoracotomies. -

Coding Billing
Coding&Billing Quarterly JANUARY 2018 Welcome to the January issue of the ATS Coding and Billing Quarterly. This issue covers a lot of new Medicare EDITOR payment policies that will impact ATS members and their ALAN L. PLUMMER, MD ATS RUC Advisor practices starting Jan. 1, 2018. ADVISORY BOARD MEMBERS: Medicare released the final rules for both the 2018 Medicare KEVIN KOVITZ, MD Chair, ATS Clinical Practice Committee Physician Fee Schedule – which covers payment and coverage KATINA NICOLACAKIS, MD policies for physicians and other Medicare Part B providers Member, ATS Clinical Practice Committee – and the Medicare Hospital Outpatient Prospective ATS Alternate RUC Advisorr Payment Rule – which deals with reimbursement STEPHEN P. HOFFMANN, MD Member, ATS Clinical Practice Committee for hospital outpatients services. While neither rule includes any major ATS CPT Advisor policy shifts that will directly impact ATS members, there are a number of MICHAEL NELSON, MD reimbursement changes (positive and negative) that will affect procedure code Member, ATS Clinical Practice Committee ATS Alternate CPT Advisor families of interest to ATS members. This issue will both alert you to these STEVE G. PETERS, MD payment changes and provide some background on why these reimbursement Member, ATS Clinical Practice Committee changes are happening. The New Year will bring in some revised CPT codes of interest to the ATS In This Issue community. This issue explains these revised CPT codes. Also covered in Revised CPT Codes for 2018, page 2 this edition is news about the revised ICD-10-CM codes for pulmonary hypertension. While the revised family of codes was in use starting Oct. -

Clinical Analysis of Pneumonectomy for Destroyed Lung: a Retrospective Study of 32 Patients
General Thoracic and Cardiovascular Surgery (2019) 67:530–536 https://doi.org/10.1007/s11748-018-01055-6 ORIGINAL ARTICLE Clinical analysis of pneumonectomy for destroyed lung: a retrospective study of 32 patients Fuat Sayir1 · Ilhan Ocakcioglu2 · Abidin Şehitoğulları3 · Ufuk Çobanoğlu1 Received: 24 September 2018 / Accepted: 19 December 2018 / Published online: 2 January 2019 © The Japanese Association for Thoracic Surgery 2019 Abstract Objective Destroyed lung is whole lung destruction secondary to chronic or recurrent lung infections. This clinical con- dition can result in irreversible changes in the lung parenchyma. In this study, we aimed to evaluate patients undergoing pneumonectomy with a diagnosis of lung destruction in terms of surgical technique, post-operative morbidity and mortality, and long-term outcomes. Methods A total of 32 patients that underwent pneumonectomy due to a destroyed lung between 2005 and 2017 were ret- rospectively reviewed. Age, gender, presenting symptoms, etiologies, localization of the destruction, pre-operative medical history, pre- and post-operative respiratory function tests, intraoperative complications and bleeding volume, morbidity and mortality, length of hospital stay, and long-term follow-up outcomes were reviewed for each patient. Results The study included 32 patients with a mean age of 31.7 ± 10.8 years. All the patients presented with persistent cough, whereas sputum production was presented by 25, hemoptysis by 18, and chest pain by 11 patients. The underlying primary diseases included nonspecific bronchiectasis in 20 (62.5%), tuberculosis in 9 (28.1%), left pulmonary hypoplasia accompanied by Bochdalek hernia in 2 (6.2%), and aspiration of a foreign body lodged in the left main bronchus in 1 (3.1%) patient. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular